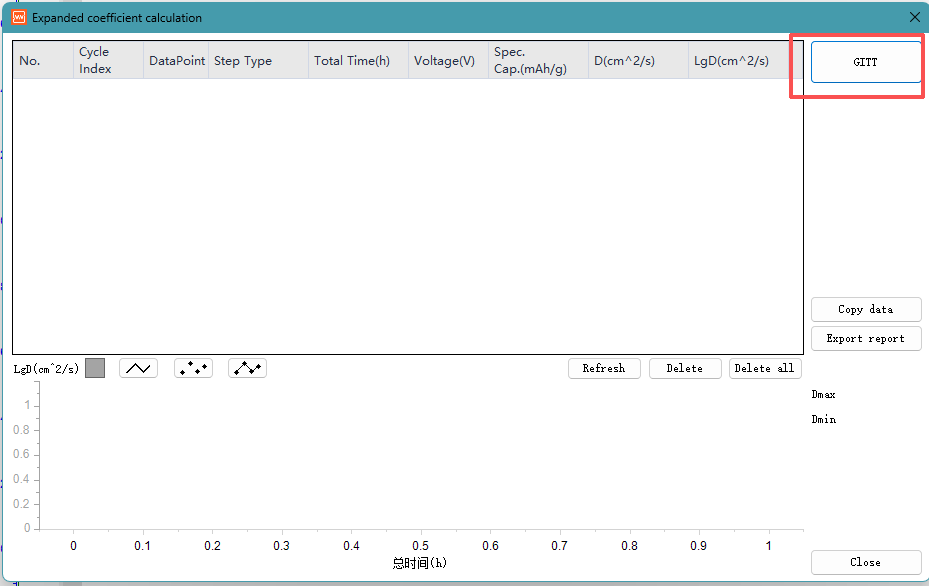
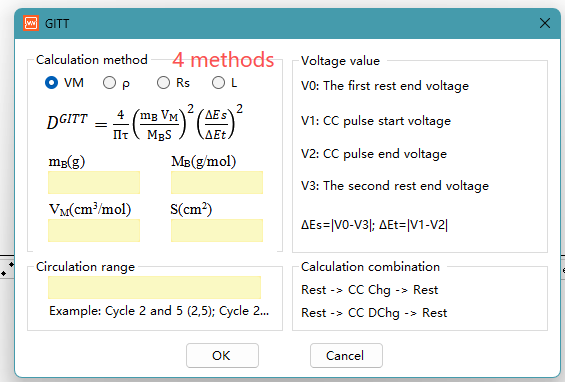
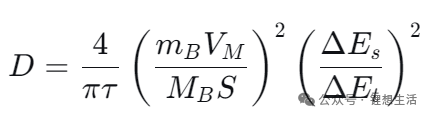Here are 4 formulas for calculating the GITT diffusion coefficient in NEWARE‘s software. GITT is Galvanostatic Intermittent Titration Technique.
Here is a brief explanation of the meaning and application scenarios of these four formulas, and the basic principles of GITT:
GITT measures how the voltage (potential) of an electrode/cell changes with the amount of charge (lithium ions, for batteries) inserted/extracted—but with intermittent “rest periods” to minimize polarization (distortions from rapid charging/discharging).
How It Works
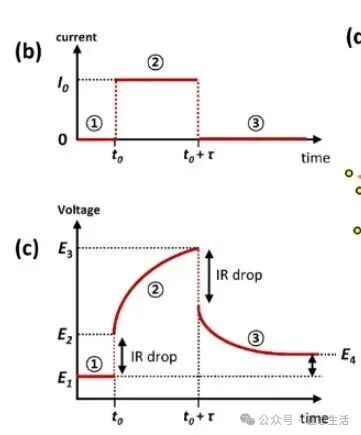
1. Galvanostatic Step: Apply a constant current (galvanostatic) to the cell for a short time. This drives ions (e.g., Li⁺) into or out of the active material, changing its state of charge (SOC).
2. Rest Period: Stop the current and let the cell “relax” (usually minutes to hours). This allows concentration gradients of ions inside the material to equalize, reducing concentration polarization (a major source of voltage error in fast tests).
3. Data Collection: Record the cell voltage continuously during both the current step and rest period. The “stable voltage” at the end of each rest period reflects the equilibrium potential of the material at that specific SOC.
4. Repeat: Cycle through these “current step + rest” sequences until the full SOC range (e.g., 0% to 100% for a battery electrode) is covered.
All GITT diffusion coefficient calculation formulas are derived from the analysis of a “current pulse-relaxation” cycle:
1. Constant current pulse: Applying a short-duration constant current causes lithium insertion or extraction in the electrode material;
2. Open-circuit relaxation: Turning off the current allows the system to relax to a new equilibrium potential;
3. Potential analysis: Calculating the diffusion coefficient D by analyzing the potential changes during the pulse and relaxation periods. That’s why it is D in NEWARE software.
Key parameters of the GITT test
D, diffusion coefficient, target for calculation (cm²/s);
t or τ, duration of the constant current pulse (s);
ΔEs steady-state potential change = |E1 – E4|, reflecting the total concentration change caused by the current pulse;
ΔEt or ΔEτ transient potential change = |E2 – E3|, reflecting the voltage that has not relaxed due to polarization during the pulse.
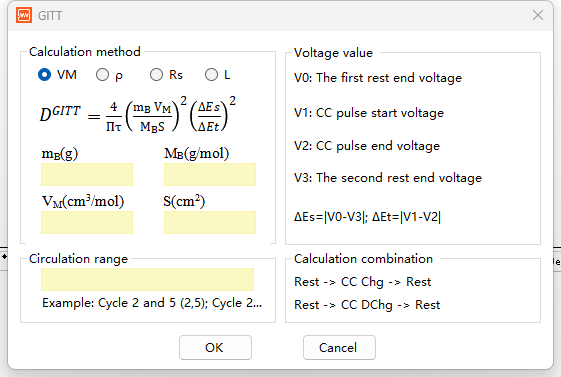
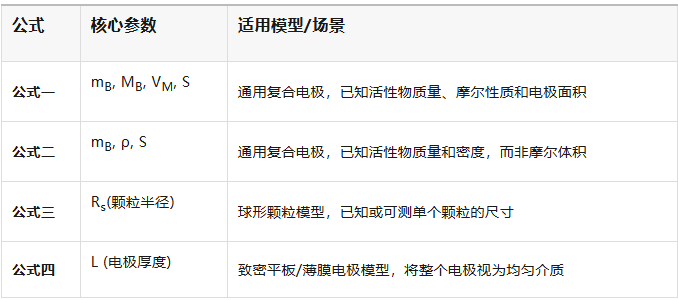
Formula 1: Based on molar volume and mass of active material
DGITT= (4/πt) × (mBVM/ MBS)2× (ΔEs/ΔEt)2
mB, mass of active material in the electrode (g)
MB, molar mass of active material (g/mol)
VM, molar volume of active material (cm³/mol)
S, contact area between electrode and electrolyte (cm²), (What does area S refer to in the GITT diffusion coefficient formula?)
The combined term (mBVM/MBS) represents the effective diffusion thickness. If S is taken as the electrode surface area, it is similar to the electrode thickness L.
Usage scenarios: This is the most classic and universal GITT diffusion coefficient formula, suitable for situations where the mass, molar mass, molar volume, and exact electrochemical active area of the active material in the electrode are clearly known. It is often used for composite electrodes with well-defined compositions.
Formula 2: Based on the density of the active material
DGITT = (4/πt) × (mB/ρS)² × (ΔEs/ΔEt)²
ρ is the apparent density of the active material (g/cm³), and the combined term (mB/ρS) represents the effective diffusion thickness.
Usage scenario: Molar volume VM = MB/ρ. Formula 2 is an equivalent form of Formula 1. This formula is more convenient when the density of the active material is known, rather than the molar volume. In practical research, these two formulas can be used interchangeably.
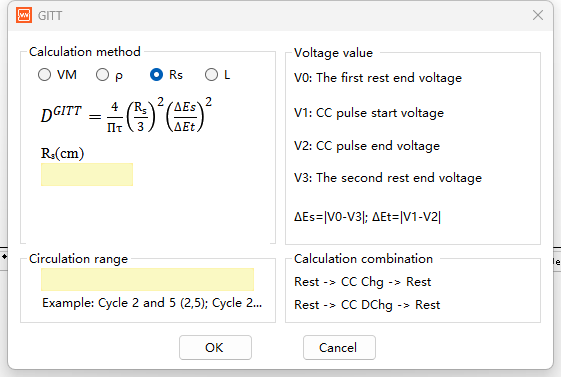
Formula 3: Based on Particle Radius
DGITT = (4/πτ) × (Rs/3)² × (ΔEs/ΔEτ)²
Rs, particle radius of the active material (cm); Rs/3, characteristic diffusion length, applicable to spherical particle diffusion models.
Use Case: Specifically applicable to spherical or near-spherical particles. This formula is most direct and accurate when the radius of the active material particles can be directly measured or is known using scanning electron microscopy (SEM), assuming that ions diffuse radially within the spherical particle.
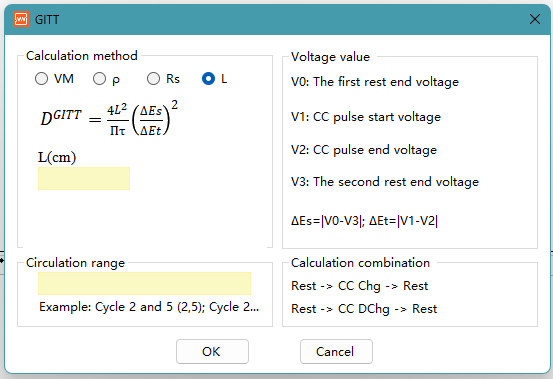
Formula 4: Based on Electrode Thickness
DGITT = (4L²/πt) × (ΔEs/ΔEt)²
L, the electrode thickness (cm), directly uses the electrode thickness L as the characteristic length of diffusion.
Use Case: The simplest formula, treating the entire electrode as a dense plate or thin film. Applicable to thin film electrodes, or electrodes where pores can be ignored and treated as a monolithic material. In this model, ions diffuse across the entire electrode thickness L in a one-dimensional direction.
How to choose a formula? The choice depends on your understanding of the electrode structure and which parameters are known and reliable:
1. If you are studying porous composite electrodes in commercial batteries, typically use formula 1 or 2;
2. If you are studying the diffusion of single particles at the material level, and the particles are spherical, use formula 3;
3. For high-quality, dense thin-film electrodes, formula 4 is the simplest choice.
Note:
1. Unit Consistency: All parameters must use centimeter-gram-second (CGS) units, or the International System of Units (SI), to ensure that the calculated diffusion coefficient D is in cm²/s or m²/s.
2. Applicability Assumptions: All formulas are based on the assumption of “small pulse, linear response.” If the pulse current is too large or the duration is too long, the calculation results may deviate significantly.
3. Relative Comparison: GITT is more commonly used to track the trend of diffusion coefficient changes with electrode potential (or lithium insertion state) for relative comparison. Its absolute value may sometimes contain errors due to inaccurate estimation of geometric parameters (such as S, L).
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
Source: WeChat 锂想生活 https://mp.weixin.qq.com/s/zZHK2YKMbpqpwPgk6gL8mA
Maybe you will be interested:
Application of Cyclic Voltammetry (CV) in Lithium Battery Research
5-Minute Guide to Neware Battery Testing System Charge/Discharge Steps