AFM: Dynamic pH regulation and interfacial ion redistribution of dendrite-free zinc metal anodes via molecular buffering
Reference link: https://doi.org/10.1002/adfm.202514260
Research Overview
Aqueous zinc-ion batteries (Zn-ion batteries) suffer from irreversible anode performance degradation caused by electrolyte alkalinization and uneven Zn deposition driven by the hydrogen evolution reaction (HER). This study proposes a bifunctional buffer additive that synergistically combines pH regulation and interfacial ion redistribution. This buffer additive maintains a self-regulated electrolyte pH (≈3) through a proton donor-acceptor equilibrium, effectively scavenging hydroxyl ions to prevent Zn passivation. It also induces preferential adsorption of Na⁺ on protrusions, promoting planar Zn deposition through a charge screening effect. This dual mechanism enables Cu-Zn half-cells to exhibit an ultralow nucleation overpotential (24.5 mV) and ultrahigh Coulombic efficiency (99.9% over 12,000 cycles). Full cells using a NaV⁃O⁃ cathode exhibit a capacity retention of 93% after 3000 cycles at a current density of 10 A g⁻¹, while pouch cells (with a cathode material of 20.89 mg cm⁻²) exhibit a capacity retention of 81.6% after 300 cycles at a current density of 1 A g⁻¹. This work establishes a universal paradigm for stabilizing zinc anodes through coupled thermodynamic and kinetic regulation, making zinc-ion batteries a viable candidate for grid-scale energy storage. The related research was published in the internationally renowned journal Advanced Functional Materials under the title “Dynamic pH Regulation and Interfacial Ion Redistribution via Molecular Buffering for Dendrite-Free Zn Metal Anodes.”
Graphic Guide
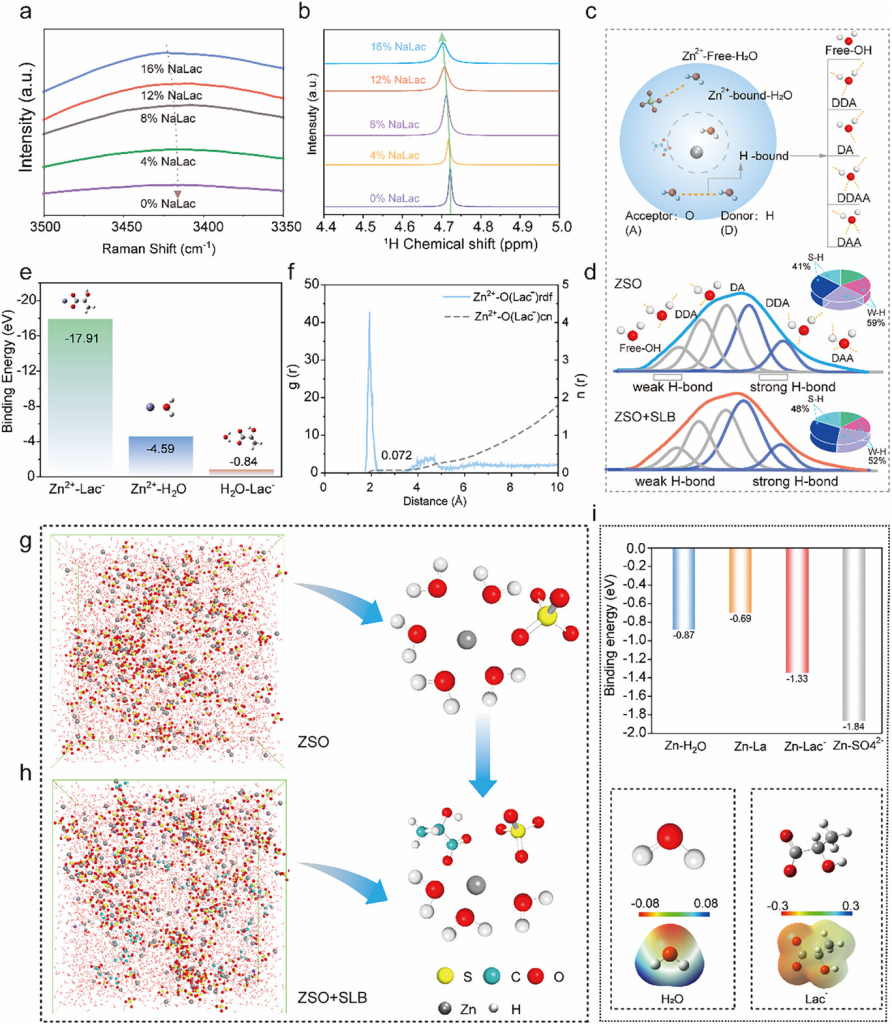
Figure 1. a) Raman spectra of electrolytes with different NaLac concentrations. b) 1H NMR spectra of electrolytes with different NaLac concentrations. c) Schematic diagram of the bulk solvation structure in ZSO, with the inset showing hydrogen bonds (H bonds) indicated by blue dashed lines and the classification of H bonds by proton donor-acceptor mechanism (D and A represent H bond donors/H and acceptors/O, respectively). d) Deconvolution of HO stretching vibrations. The pie chart shows the proportion of local weak hydrogen bonds/W–H (high wavenumber) and strong hydrogen bonds/S–H (low wavenumber). e) Comparison of binding energies between Zn⁺HO, Zn⁺-Lac−, and HO-Lac− systems based on DFT calculations. f) Radial distribution functions (RDFs) and coordination numbers (CNs) of Zn⁺-O(H₂O) and Zn⁺-O(Lac−) in baseline (ZSO) and modified (ZSO+SLB) electrolytes obtained from MD simulations. g,h) 3D snapshots of the electrolyte and typical solvation structures in ZSO and ZSO+SLB electrolytes obtained from MD simulations. i) Adsorption energies of electrolyte components (H₂O, NaLac, Lac−, SO₄−) on the Zn surface and electrostatic potential maps of H₂O and Lac− molecules.
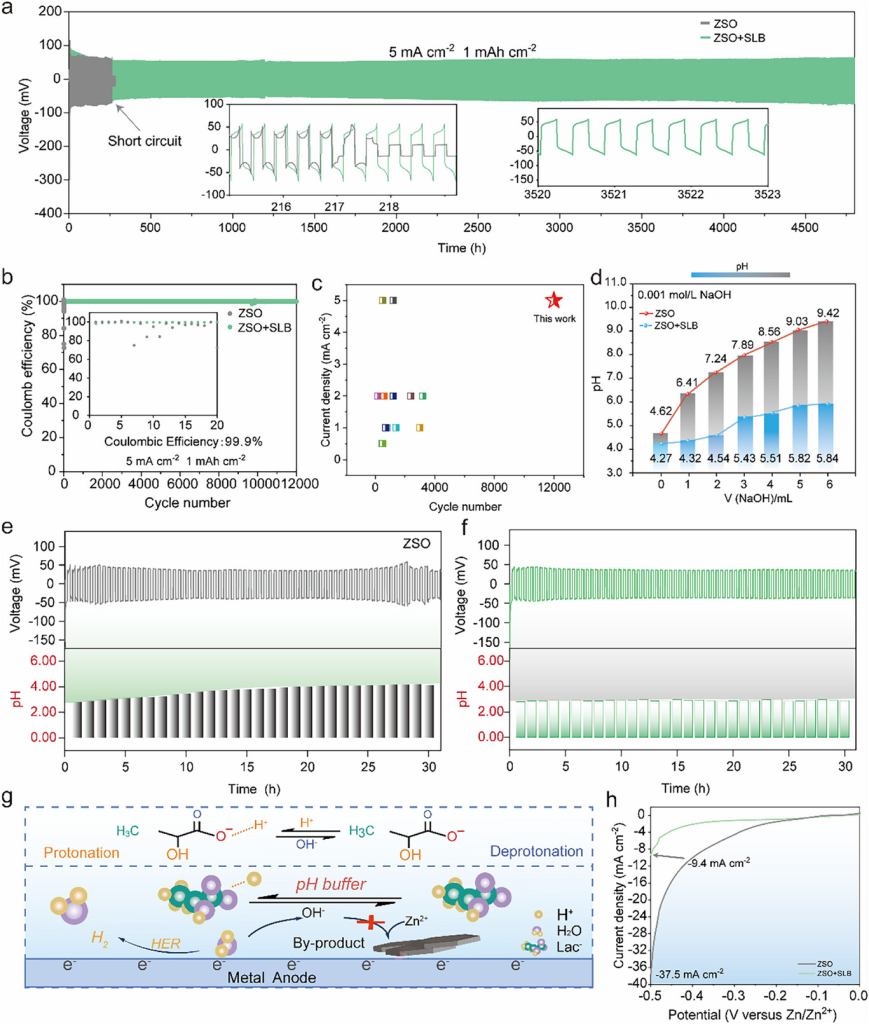
Figure 2. a) Cycling performance of a Zn-Zn symmetric cell in ZSO and ZSO+SLB electrolytes at 5 mA cm−2 and 1 mAh cm−2. b) Coulombic efficiency measurements of a Zn-Cu half-cell in ZSO and ZSO+SLB electrolytes at 5 mA cm−2 and 1 mAh cm−2. c) Comparison of the CE of the ZSO+SLB electrolyte with previously reported aqueous electrolytes. d) Titration curves of ZSO and ZSO+SLB electrolytes. e,f) In situ pH monitoring of the Zn-Zn symmetric cell in e) ZSO and f) ZSO+SLB during cycling at 1 mA cm−2 and 1 mAh cm−2. g) Schematic diagram of the principle for suppressing the hydrogen evolution side reaction. h) Linear polarization curves in different electrolytes.
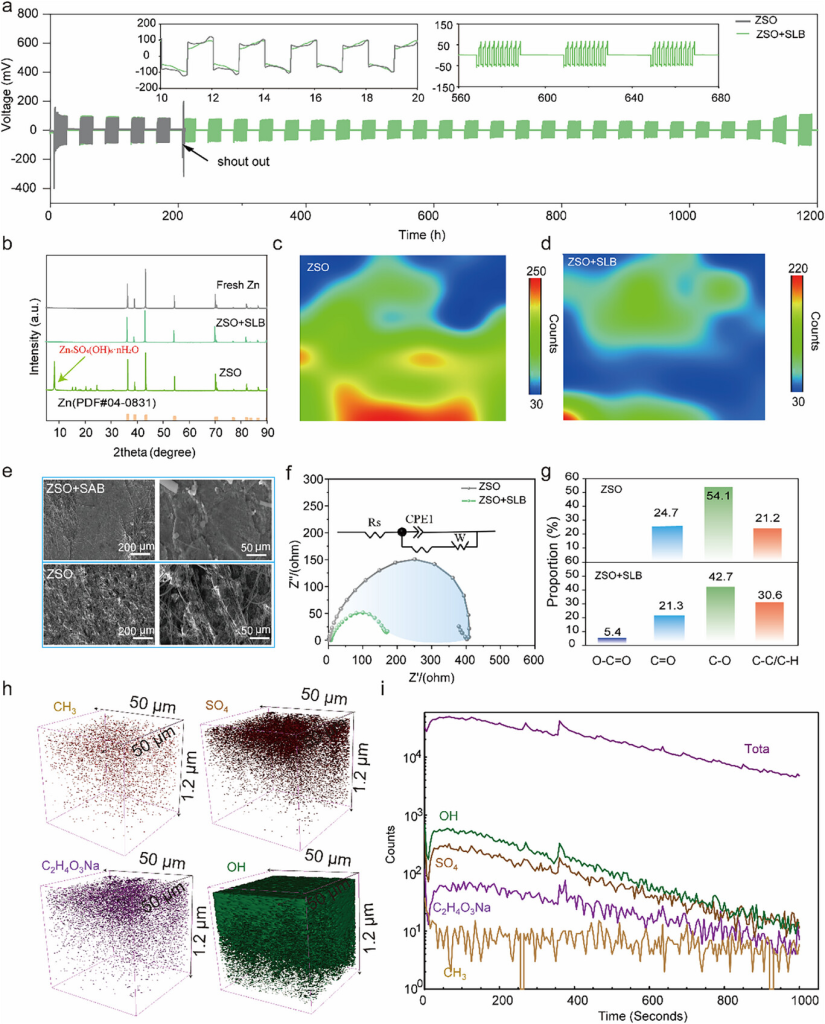
Figure 3. a) Galvanostatic cycling of a Zn-Zn battery in ZSO and ZSO+SLB electrolytes at a fixed 24-hour interval. b-e) XRD, Raman mapping, and SEM images of a Zn anode immersed in ZSO and ZSO+SLB electrolytes for 10 days. f) EIS of a symmetric Zn-Zn battery in ZSO and ZSO+SLB electrolytes. g) Relative content of various C-containing species. h,i) TOF-SIMS 3D chemical mapping of a cycled Zn anode showing the spatial distribution of key SEI components: SO42− (inorganic debris), CH3− (organic debris), C2H4O3Na (lactic acid derivative), and OH− (hydroxyl group).
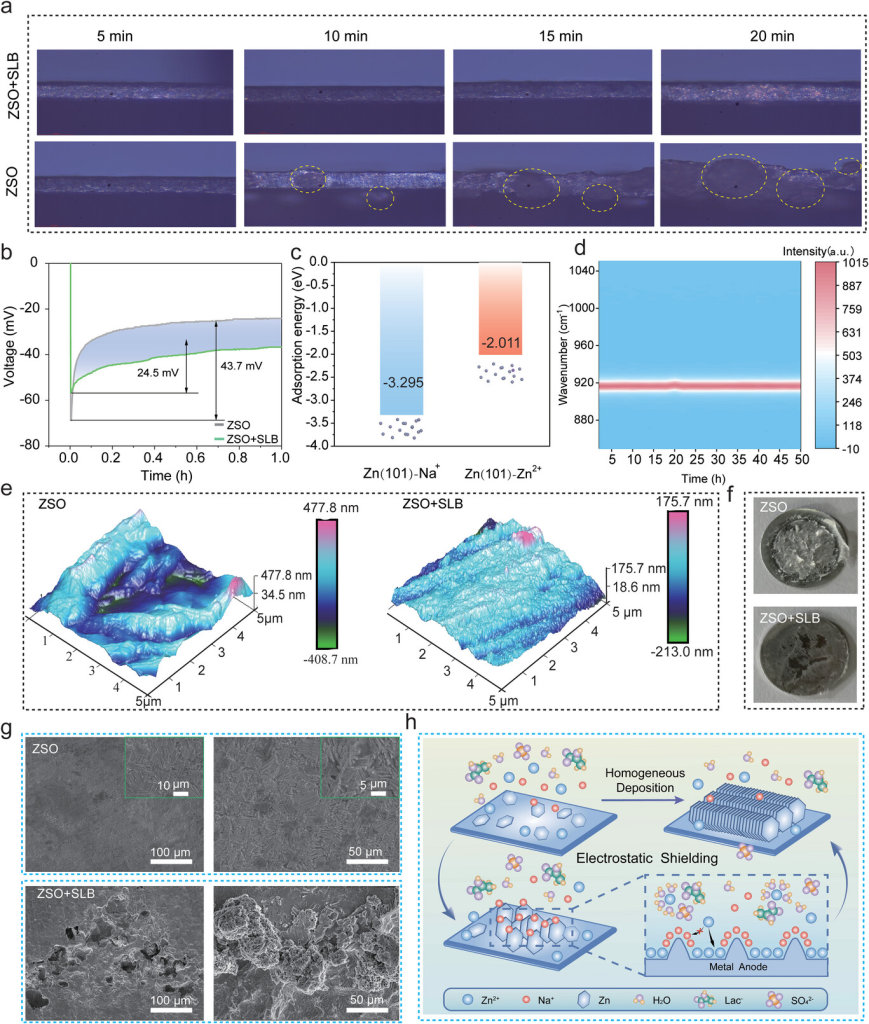
Figure 4. a) In situ optical images of Zn deposition in Zn//Zn symmetric cells in ZSO and ZSO+SLB electrolytes. b) Voltage curves of Zn-Cu half-cells in ZSO and ZSO+SLB electrolytes at 5 mA cm−2. c) Adsorption energies of Na⁺ and Zn⁺ on the Zn (101) plane. d) In situ Raman spectra of ZSO+SLB electrolyte in Na–Na symmetric cells. e) AFM images of the Zn anode after 50 cycles in ZSO and ZSO+SLB electrolytes. f) Optical images of the Zn anode after 50 cycles in ZSO and ZSO+SLB electrolytes. g) SEM images of the Zn anode after 50 cycles in ZSO and ZSO+SLB electrolytes. h) Schematic diagram of Zn deposition and interface chemistry in ZSO+SLB electrolyte.
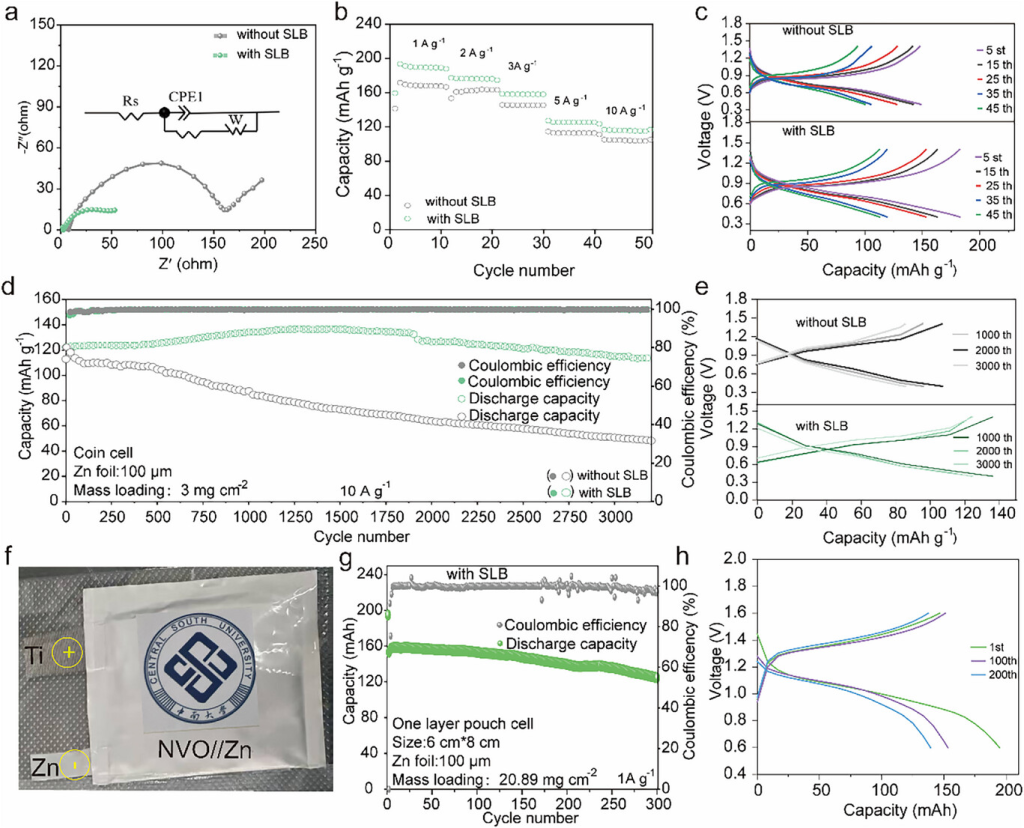
Figure 5. a) EIS curves of Zn-NVO batteries in Zn(OTF)2 and Zn(OTF)2+SLB electrolytes. b,c) Rate performance and corresponding charge-discharge curves of Zn-NVO batteries in Zn(OTF)2 and Zn(OTF)2+SLB electrolytes. d,e) Cycling stability and corresponding charge-discharge curves of Zn-NVO batteries in Zn(OTF)2 and Zn(OTF)2+SLB electrolytes at 10 A g−1. f) Photograph of the NVO-Zn pouch cell. g,h) Cycling stability and corresponding charge-discharge curves of the Zn-NVO pouch cell in Zn(OTF)2+SLB electrolyte at 1 A g−1.
Research Conclusions
This study proposes a pH-buffered electrolyte system that can simultaneously address the dual challenges of hydrogen evolution and dendrite growth that persist in aqueous Zn-ion batteries. By establishing a self-regulating pH (≈3) buffer to neutralize hydroxyl species and implementing dynamic Na⁺-mediated electrostatic screening at protrusion sites, the SLB additive achieves unprecedented interfacial stability through coupled thermodynamic and kinetic control. This synergistic mechanism enables record-breaking electrochemical performance: an ultralow nucleation overpotential of 24.5 mV, near-theoretical Coulombic efficiency (99.9% retention after 12,000 cycles), and exceptional cycling performance in both symmetric (>4,700 h) and full-cell configurations (93% capacity retention after 3,000 cycles at a current density of 10 A g⁻¹). The industrial feasibility of this system is demonstrated by high-mass loading pouch cells (20.89 mg cm⁻¹) that retain 81.6% of their capacity after 300 cycles. Beyond directly enhancing performance, this study reveals how electrolyte additives actively participate in bulk pH homeostasis and interfacial ion redistribution processes, establishing fundamental design principles for next-generation metal batteries. The bifunctional SLB strategy—simultaneously addressing HER-induced corrosion, ZnSO4 passivation, and dendritic failure—provides a general framework for stabilizing active metal anodes in aqueous systems. Crucially, the buffer-mediated ion flux regulation mechanism transcends zinc chemistry and provides generalizable insights into dynamic interface engineering for lithium, sodium, and other multivalent metal batteries. By integrating molecular-level electrolyte design with practical device engineering, this study positions zinc-ion batteries as a technologically mature candidate for grid-scale energy storage and charts a roadmap for electrolyte innovation in post-lithium energy storage systems.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
If you do battery research or battery materials research, you might be interested in these:
Neware battery cyclers for coin, pouch cells
Neware all in one for coin cells
Related News:
- Sustained Release of Underpotential Deposition Initiators for Ah-Level Zinc Metal Batteries
- A Bifunctional Separator with Gradient Distribution of MCM-41 Zeolite for High-Performance Aqueous Zinc-ion Batteries
- Lithium Ion vs Lithium Polymer: A Comprehensive Comparison Guide for 2024
- Power Battery Tech: Latest Advances & Future Trends