Brief Overview of Sodium-Ion Battery (SIB) Material Research
I. Working Principle of Sodium-Ion Batteries (SIBs)
Charge and Discharge Reactions:
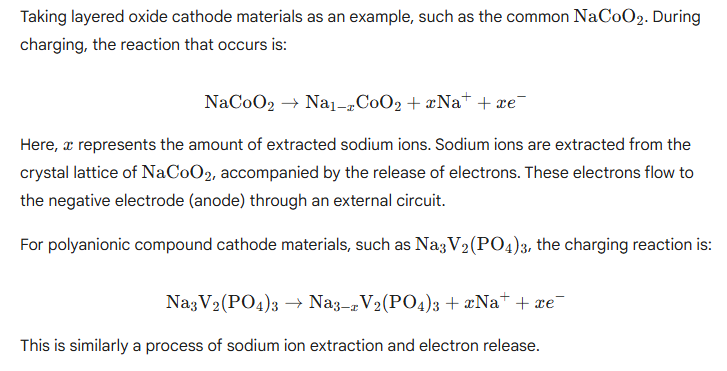
Negative Electrode (Anode): Typically Graphite (or Hard Carbon)
Charging: Sodium ions extracted from the positive electrode are intercalated into the negative electrode graphite.
Discharging: The sodium ions intercalated during charging are released and re-intercalated into the positive electrode, forming a complete current circuit.
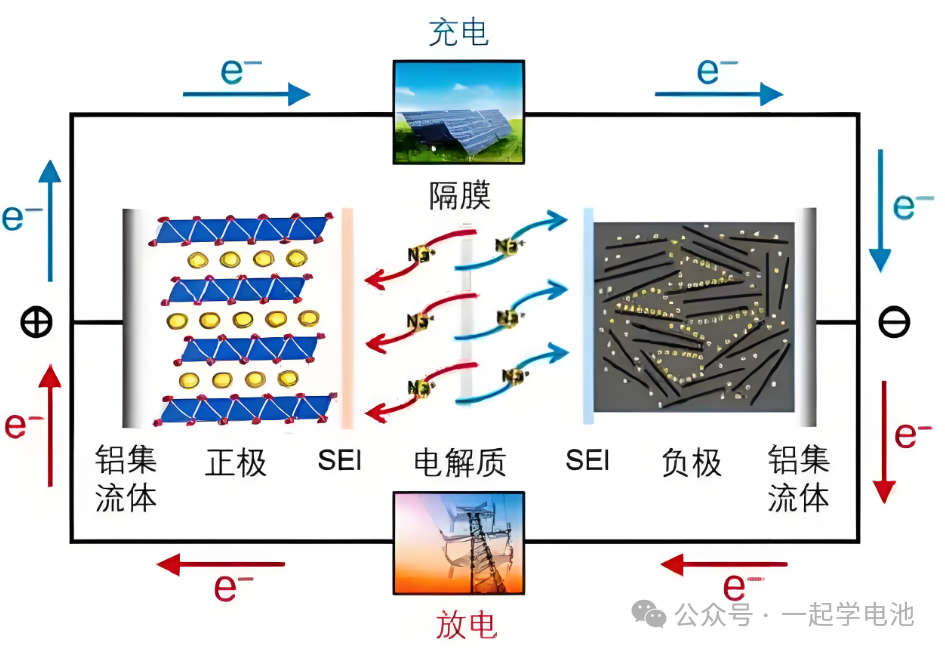
II. Introduction to SIB Components
1. Cathode Materials of SIB
Common cathode materials for sodium-ion batteries include Sodium Cobalt Oxide (NaCoO₂), Sodium Vanadium Phosphate (Na₃V₂(PO₄)₃), and Prussian Blue (NaFe[Fe(CN)₆]), among others. The cathode material is the key component for storing and releasing sodium ions within the battery.
Requirements for Cathode Materials:
① High Capacity: Must allow for the intercalation and extraction of a large number of sodium ions.
② High Voltage: Must possess a high redox potential.
③ Structural Stability: Must provide abundant channels for sodium ion transport while maintaining minimal volume change during charge/discharge cycles.
④ High Conductivity: Must exhibit both high ionic and electronic conductivity.
⑤ Chemical Stability: Must remain stable within the electrolyte.
⑥ Economic & Safety Factors: Low cost, ease of preparation, environmental friendliness, and high safety.
| No. | Physical Property Parameters | Battery Cell Performance |
| 1 | Particle Size Distribution, Particle Structure | Compacted Density, Kinetic Performance (C-rate, Low Temperature) |
| 2 | Specific Surface Area (SSA) | Kinetic Performance (C-rate, Low Temperature), High-Temperature Performance, Safety Performance |
| 3 | Tap Density | Compacted Density |
| 4 | Modification Elements (Doping/Coating) | Specific Capacity, Cycle Life, High-Temperature Performance, Safety Performance, etc. |
| 5 | Magnetic Impurity Content | Self-discharge, Safety Performance |
Types of Cathode Materials of SIB
Cathode materials for sodium-ion batteries are classified by their structural types as follows:
1. Layered Oxides (Similar to Ternary Lithium)
Primarily composed of nickel-manganese-iron-based oxides, these are prepared via solid-state or co-precipitation methods. These materials offer higher energy density and are suitable for two- or three-wheeled electric vehicles and low-speed electric vehicles.
2. Polyanionic Compounds (High Safety, similar to LFP)
Represented by Sodium Iron Phosphate (NaFePO4), conductivity is enhanced through carbon coating technology. They feature a long cycle life of over 8,000 cycles, making them ideal for energy storage scenarios.
3. Prussian Blue Analogs (Low Cost)
Based on ferrocyanides, these are produced through simple precipitation or thermal decomposition methods. While these materials have a lower cost, their cyclic stability is relatively weak. They are mostly used in cost-sensitive sectors, such as start-stop batteries.
Summary
The shortcomings of the three major cathode materials for Sodium-ion batteries:
Safety issues similar to those of ternary lithium batteries;
Low specific capacity;
Adverse effects caused by crystal water (lattice water).
Current Market Status: Currently, layered oxides are the most widely used in commercial applications, while polyanionic compounds are rapidly gaining momentum and catching up.
Performance of Cathode Materials of SIB
The cathode materials for sodium-ion batteries that have undergone commercialized research include High-Nickel Layered Oxides (NCM811), Sodium Vanadium Phosphate (Na3V2(PO4)3), and Prussian Blue (NaFe[Fe(CN)6]), among others. Currently, the materials that have reached large-scale production are primarily Layered Oxides and Polyanionic Compounds.
Performance Characteristics of the Three Major Cathode Materials
| Performance Indicators | Layered Oxides | Polyanionic Compounds | Prussian Blue |
| Specific Capacity (mAh/g) | 160+ | 117-154 | 150 |
| Avg. Working Voltage (V) | 2.7-3.5 | 2.8-3.8 | 3.0-3.4 |
| Compacted Density (g/cm³) | 3.4-3.8 | 2.8-3.2 | 1.6-2.0 |
| Energy Density (Wh/kg) | 500-600 | 400-500 | 400-500 |
| Cycle Life | 1,000-3,000 | 3,000-10,000 | 1,000-3,000 |
| Rate Performance | Excellent | Good | Good |
| Cost & Safety | High cost, Moderate safety | High/Moderate cost, High safety | Extremely low cost, High safety |
2. Negative Electrode (Anode) Materials of SIB
Common anode materials for sodium-ion batteries include hard carbon, alloys, metal oxides, and sodium metal. Similar to lithium-ion batteries, the specific capacity of anode materials is much higher than that of cathode materials. Therefore, the primary considerations for anode materials are cost and stability.
Requirements for Anode Materials:
① Appropriate Sodium Intercalation Potential: Must have a suitable potential for sodium ion insertion.
② Excellent Stability: Must maintain performance over time.
③ Structural Integrity: Must provide abundant channels for sodium ion extraction and intercalation, with minimal volume change during charge/discharge cycles.
④ High Conductivity: Must exhibit high ionic and electronic conductivity.
⑤ Optimized Texture: Must have appropriate pore structures and surface characteristics.
⑥ Economic & Safety Factors: Low cost, ease of preparation, environmental friendliness, and high safety.
Table 1: Physical Property Parameters vs. Battery Cell Performance
| No. | Physical Property Parameters | Battery Cell Performance |
| 1 | Particle Size Distribution, Particle Structure | Compacted Density, Kinetic Performance (Rate Capability, Low Temperature) |
| 2 | Specific Surface Area (SSA) | Kinetic Performance (Rate Capability, Low Temperature), High-Temperature Performance, Safety Performance |
| 3 | Tap Density | Compacted Density |
| 4 | Modification Elements | Specific Capacity, Cycle Life, High-Temperature Performance, Safety Performance, etc. |
| 5 | Magnetic Impurity Content | Self-discharge, Safety Performance |
Note: Dendrite formation is a more severe issue in sodium-ion batteries than in lithium-ion batteries; therefore, the selection of the potential for anode materials must be strictly controlled.
Performance of Anode Materials
Performance Characteristics of the Four Major Anode Materials:
| Performance Indicator | Hard Carbon | Alloy-based | Metal Oxides | Sodium Metal |
| Specific Capacity (mAh/g) | 250-350 | 600-2600 | 150-300 | 1166 |
| Avg. Potential (V) | 0.1-0.3 | 0.3-0.8 | 0.3-1.0 | 0 |
| Advantages | Best overall performance | High specific capacity | Most stable | Lowest potential |
| Disadvantages | Volumetric energy density | Poor cycling | Poor conductivity | Safety issues |
| Industrialization | Mainstream | R&D stage | Phased out | Laboratory |
3. Electrolyte
Electrolyte: The electrolyte is the medium for sodium ion transport within sodium-ion batteries. It typically consists of organic solvents (such as carbonates, carbamides, etc.) and sodium salts (such as sodium fluoride, sodium hexafluorophosphate, etc.).
SEI Film (Solid Electrolyte Interphase): A passivation layer formed during the first cycle of a sodium battery by a passivation reaction occurring at the solid-liquid phase interface between the electrolyte and the anode material. This passivation layer is an interfacial layer that possesses the characteristics of a solid electrolyte.
| Electrolyte Components | Common Types |
| Solvents | EC, PC, DMC, DEC, EMC |
| Sodium Salts | NaPF6, NaClO4 |
| Additives | Film-forming, Flame retardant, Overcharge protection, Water removal |
III. Improvements in SIB — Cathode Material Modification
The layered oxides and polyanionic compounds in sodium-ion battery cathode materials share similar advantages and disadvantages with lithium-ion battery cathode materials (Ternary and LFP). The primary issue for Prussian Blue-type materials lies in the difficulty of removing crystal water. Consequently, the research and improvement of these three cathode materials have received widespread attention.
Methods for Cathode Material Modification:
1. Doping: Layered oxides can balance energy density and cyclic stability through doping and adjusting the ratio of transition metals. However, attention must be paid to phase transition issues of different metals during charging and discharging.
2. Coating: Polyanionic materials can enhance electronic conductivity and suppress side reactions by coating a carbon layer on the material surface. Nevertheless, coating can impact the specific capacity of the battery.
3. Drying: For Prussian Blue analogs, partial crystal water can be removed through vacuum drying as a post-synthesis treatment. However, excessively high temperatures can cause irreversible damage to the structure.
4. Composite Modification: This involves simultaneously using multiple modification methods, such as doping and coating, to improve ion transport kinetics.
IV. Material Characterization
What is Material Characterization? Material characterization involves using various physical and chemical testing methods to reveal and determine the structural characteristics of a material. By utilizing the interaction of light beams, electron beams, or other particles with the sample, various types of information representing the material’s structural features are generated, providing rich data on morphology, composition, and structure.
Key Characterization Techniques:
1. Scanning Electron Microscopy (SEM): Used to observe the surface morphology and microstructure of materials, providing high-resolution images.
2. Transmission Electron Microscopy (TEM): Used to observe the internal microstructure of materials, capable of providing even higher resolution images and crystal structure information.
3. X-ray Diffraction (XRD): Used to analyze the crystal structure of materials and determine lattice parameters and crystal structure types.
4. Fourier Transform Infrared Spectroscopy (FTIR): Used to analyze chemical composition and chemical bond information, which can determine the functional groups and molecular structure of the material.
5. Thermogravimetric-Differential Thermal Analysis (TG-DTA): Used to measure mass changes and thermal properties of materials during heating, including thermal weight loss and thermal reaction characteristics.
6. Raman Spectroscopy (Raman): Used to analyze molecular and lattice vibrations, providing information regarding the material’s structure and composition.
These techniques help researchers gain a deep understanding of material structure, composition, and performance, providing a crucial experimental basis for the design, preparation, and application of materials.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
