Electrochemical Impedance Spectroscopy (EIS)
Electrochemical impedance spectroscopy (EIS) is a robust method for examining how AC impedance varies with frequency in an electrochemical system that’s in a polarization steady state, usually at equilibrium potential. This technique offers crucial insights into electrochemical processes like charge transfer resistance, electrolyte resistance, and double-layer capacitance within the system.
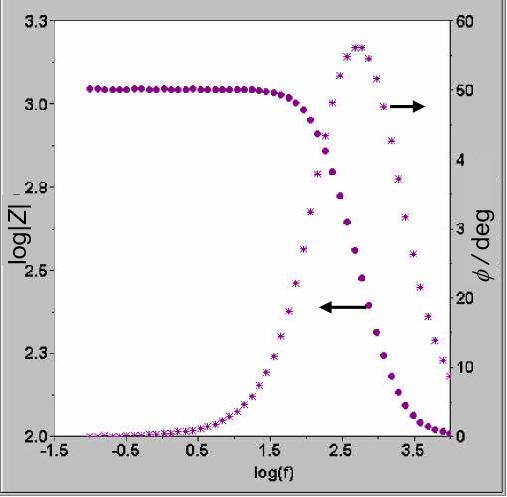
During EIS testing, several challenges may emerge, necessitating appropriate solutions:
1. **Electrode Polarization**: Electrode polarization can notably impact EIS measurements. It can stem from various factors, such as electrolyte resistance, charge transfer resistance, and double-layer capacitance. To counteract this, applying proper calibration methods, like subtracting the electrode polarization contribution from the impedance data, is vital for accurate results.
2. **Experimental Setup**: The setup for EIS testing must be meticulously designed to minimize parasitic elements that can cause measurement errors. Factors like cable capacitance, contact resistance, and stray capacitance can compromise impedance measurement accuracy. Using appropriate shielding, precise electrode placement, and high-quality cables and connectors can help mitigate these issues.
3. **Frequency Range and Resolution**: Choosing the right frequency range and resolution is key to obtaining meaningful and accurate impedance spectra. The range should encompass the relevant electrochemical processes, and the resolution should be adequate to capture impedance changes in detail. An overly broad or narrow range may lead to insufficient sensitivity or loss of important information. Thus, understanding the system’s characteristics and expected processes is essential for determining the optimal frequency range and resolution.
4. **Data Interpretation**: Interpreting EIS-measured impedance spectra requires a solid grasp of electrochemical principles. Typically, these spectra are fitted to equivalent circuit models to extract meaningful information about the system’s properties and processes. Accurate interpretation and parameter extraction hinge on proper model selection and fitting techniques.
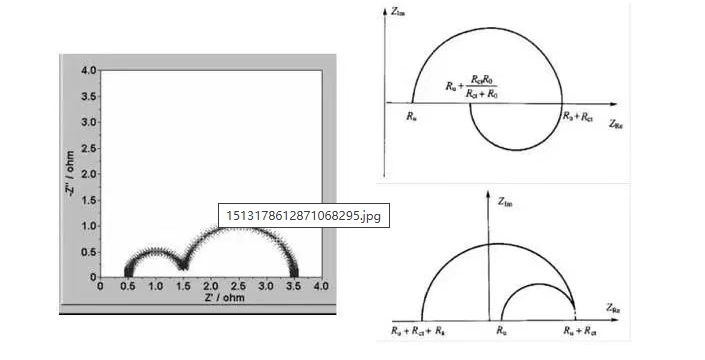
The value of electrochemical impedance spectroscopy lies in its capacity to offer a comprehensive understanding of the electrochemical processes within a system. EIS allows for the determination of various electrochemical parameters, including charge transfer resistance, diffusion coefficients, and reaction kinetics. It aids in characterizing electrochemical interfaces, evaluating system performance, and identifying degradation mechanisms.
EIS is utilized across multiple fields, such as battery research, fuel cell analysis, corrosion studies, and sensor development. It helps optimize electrode materials, investigate electrochemical reaction mechanisms, and develop efficient energy storage and conversion devices. EIS also assesses how environmental factors like temperature, humidity, and gas composition affect electrochemical systems.
In conclusion, electrochemical impedance spectroscopy is an invaluable tool for studying AC impedance variation with frequency in an electrochemical system under a polarization steady state. By addressing potential issues and employing suitable data analysis techniques, this method enhances our understanding of electrochemical processes, system optimization, and the advancement of cutting-edge electrochemical technologies.
This is a brief introduction to EIS from Google Gemini. What is EIS?
At its core, EIS is a frequency-domain analysis. While a standard discharge test tells you how much energy a battery holds, EIS tells you why the battery might be underperforming.
We apply a small AC stimulus (usually a voltage sine wave, \Delta V) across a range of frequencies and measure the current response (\Delta I). By calculating the ratio, we get the Impedance (Z), which is essentially “resistance” that changes depending on how fast you toggle the current.
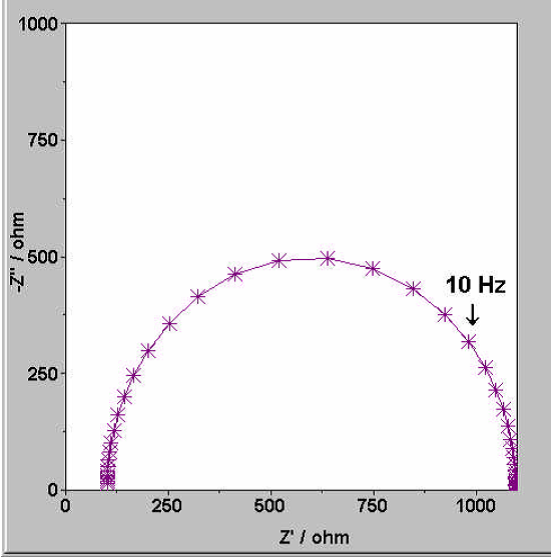
The Nyquist Plot: The Battery’s Fingerprint
When you look at an EIS report, you’ll most often see a Nyquist Plot. It plots the Real Impedance (Z’) against the Imaginary Impedance (-Z”). A typical lithium-ion battery plot consists of several distinct regions:
Ohmic Resistance (R_s): The point where the curve intercepts the x-axis at high frequencies. This represents the resistance of the electrolyte, tabs, and current collectors.
The Semi-circle: This represents Charge Transfer Resistance (R_{ct}) and the Double Layer Capacitance. It tells us how hard it is for lithium ions to cross the interface between the electrolyte and the electrode.
Scientist’s Note: If this circle grows over time, your battery is likely forming a thick SEI (Solid Electrolyte Interphase) layer, which degrades performance.
The Tail (Warburg Impedance): The 45-degree sloped line at low frequencies. This represents diffusion—how fast the ions can physically move through the active material.
Why do we use it?
As scientists, we use EIS for three main “Health Checks”:
State of Health (SoH) Monitoring: We can see internal degradation long before the battery actually “dies.”
Separating Mechanisms: Unlike a simple multimeter, EIS allows us to distinguish between “liquid” problems (electrolyte) and “solid” problems (electrode cracking).
Temperature Sensitivity: We use EIS to study how batteries behave in extreme cold, where diffusion becomes the bottleneck.
Alternating current voltammetry
AC Voltammetry is a method employed to examine the correlation between the amplitude and phase of AC current and the DC polarization potential at a given frequency. This technique offers crucial insights into the electrochemical processes taking place at the electrode-electrolyte interface.
The significance of AC Voltammetry is rooted in its capacity to elucidate electrochemical processes at the electrode-electrolyte interface. By probing the relationship between AC current amplitude, phase, and DC polarization potential, it allows for the determination of various electrochemical parameters. These include charge transfer kinetics, double-layer capacitance, and faradaic processes.
AC Voltammetry is utilized across a wide range of fields, such as corrosion studies, electroplating, battery characterization, and sensor development. It aids in evaluating electrode performance, optimizing electrochemical processes, and understanding the mechanisms of electrochemical reactions. AC Voltammetry also supports the development of new materials and technologies for energy storage, electrocatalysis, and electrochemical sensing.
In conclusion, AC Voltammetry is an invaluable tool for investigating the relationship between AC current characteristics and DC polarization potential at a specific frequency. By addressing potential issues and employing suitable data analysis techniques, this method enhances our understanding of electrochemical processes, system optimization, and the advancement of cutting-edge electrochemical applications.
For more battery and battery testing knowledge, please visit the website NEWARE.
