How do aqueous zinc-ion batteries relate to traditional Zn//MnO₂ electrochemical systems?
Understanding the Link: Traditional Zn//MnO₂ Batteries vs. Modern Aqueous Zinc-ion Batteries (AZIBs)
In the rapidly evolving world of energy storage, the “Zinc-Manganese” system is experiencing a significant renaissance. While most consumers are familiar with the classic Zn//MnO₂ alkaline battery used in household remotes, researchers are now pivoting toward the Aqueous Zinc-ion Battery (AZIB) as a sustainable alternative to Lithium-ion.
What is an Aqueous Zinc-ion Battery (AZIB)?
An Aqueous Zinc-ion Battery (AZIB) is a type of secondary (rechargeable) energy storage system that utilizes zinc ions ($Zn^{2+}$) as the charge carriers, moving between a zinc metal anode and an intercalation cathode through a water-based (aqueous) electrolyte.
Unlike traditional lithium-ion batteries that use flammable organic solvents, AZIBs are highly regarded for their intrinsic safety, low cost, and environmental friendliness.
Key Components and Working Mechanism
The architecture of a typical AZIB consists of:
Anode: Zinc metal foil (Zn), which offers high theoretical capacity (820 mAh/g) and a low redox potential.
Cathode: Usually manganese-based (MnO2), vanadium-based, or Prussian blue analogs that can reversibly host Zn2+ ions.
Electrolyte: An aqueous solution of zinc salts (e.g., ZnSO4 or Zn(CF3SO3)2).
Mechanism: It follows a “rocking-chair” principle. During discharge, Zn2+ ions strip from the anode and intercalate into the cathode’s crystal lattice; the process reverses during charging.
Advantages of Aqueous Zinc-ion Battery (AZIB)
Safety: The water-based electrolyte is non-flammable and non-explosive.
Cost-Effectiveness: Zinc is earth-abundant and much cheaper than lithium or cobalt.
High Power Density: The fast ionic conductivity of aqueous electrolytes allows for rapid charging/discharging.
Environmental Impact: Zinc is non-toxic and easier to recycle than heavy metals used in other battery chemistries.
But what exactly is the relationship between Traditional Zn//MnO₂ Batteries and Modern Aqueous Zinc-ion Batteries (AZIBs)? Are they the same technology, or entirely different species?
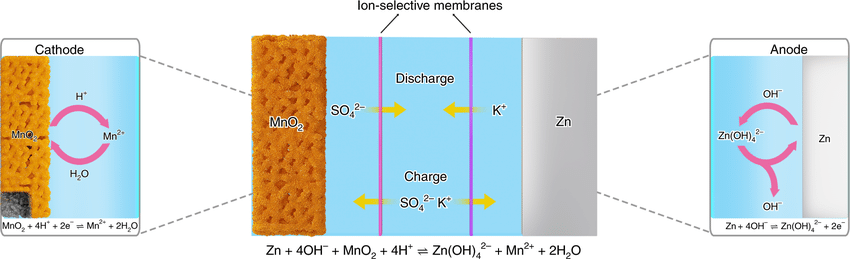
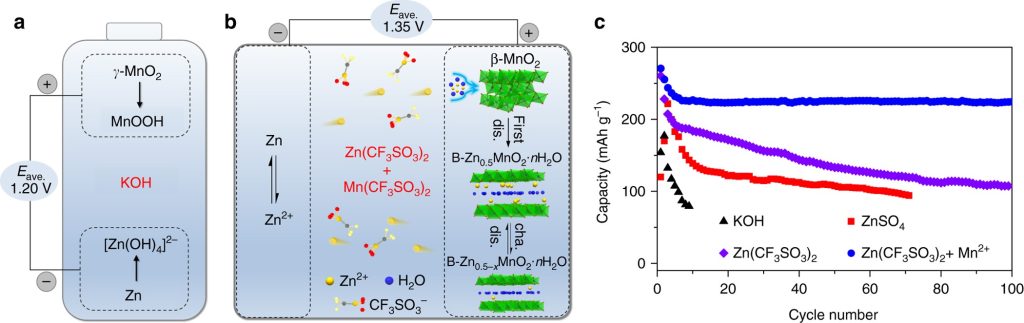
1. The Heritage: From Primary to Secondary Cells
At its core, the relationship is one of evolution. The traditional Zn//MnO₂ battery is a primary (non-rechargeable) battery, while the Aqueous Zinc-ion Battery is its secondary (rechargeable) successor.
Historically, zinc-manganese batteries dominated the market due to their low cost and safety. However, the move toward “Zinc-ion” technology represents a shift from a simple one-way chemical reaction to a sophisticated, reversible ion-intercalation process.
2. The Game Changer: Alkaline vs. Mildly Acidic Electrolytes
The fundamental difference lies in the electrolyte chemistry, which dictates whether the battery can be recharged.
Traditional Zn//MnO₂ (Alkaline): Uses a strong base like Potassium Hydroxide (KOH). In this harsh environment, the chemical reactions are irreversible. The discharge products form complex insulators that prevent the battery from being charged back to its original state.
Aqueous Zinc-ion Battery (AZIB): Switches to a neutral or mildly acidic aqueous electrolyte (e.g., ZnSO4 or Zn(CF3SO3)2). This change in pH allows Zinc ions (Zn2+) to move back and forth between the electrodes without destroying the material structure.
Test your aqueous zinc-ion batteries
3. The Mechanism: Dissolution vs. Intercalation
The way energy is stored differs significantly between the two systems:

4. Why Manganese Dioxide (MnO2)?
In both systems, MnO2 is the preferred cathode material because of its polymorphic nature. MnO2 exists in various crystal structures (e.g., alpha, beta, gamma, delta phases) that provide “tunnels” or “layers.”
In AZIBs, researchers specifically target structures like alpha-MnO2 (tunnel-type) or delta-MnO2 (layered-type) because their atomic spacing is wide enough to allow Zn2+ ions to “shuttle” in and out efficiently during charge and discharge cycles.
5. Conclusion: Why This Matters for the Future
The Aqueous Zinc-ion Battery is effectively the “Rechargeable 2.0” version of the classic dry cell. By utilizing the low cost of Zinc and the safety of water-based electrolytes, AZIBs are currently a leading candidate for large-scale grid energy storage.
While challenges like “zinc dendrite growth” and “manganese dissolution” remain, the transition from the old Zn//MnO₂ system to the new AZIB platform is a crucial step toward a greener, safer, and cheaper battery future.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, or EV driving simulation test, please feel free to contact us.
Related News:
- Zn//MnO2 battery from Primary to Rechargeable: A Timeline of Key Breakthroughs in Aqueous Zinc-Ion Batteries (AZIBs) 2026 post
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-2
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-1
- A Bifunctional Separator with Gradient Distribution of MCM-41 Zeolite for High-Performance Aqueous Zinc-ion Batteries
