Lithium Plating in Lithium-ion Batteries: Causes and Manifestations
I. Why Does Lithium Plating Occur?
In simple terms, when the anode cannot promptly accommodate or intercalcate lithium ions migrating from the cathode, these ions “pile up” on the surface of the anode and are reduced into metallic lithium. As this deposited lithium continues to accumulate, it evolves into lithium dendrites. These dendrites typically manifest in four distinct morphologies: whisker-like, mossy, dendritic (tree-like), and spherical.
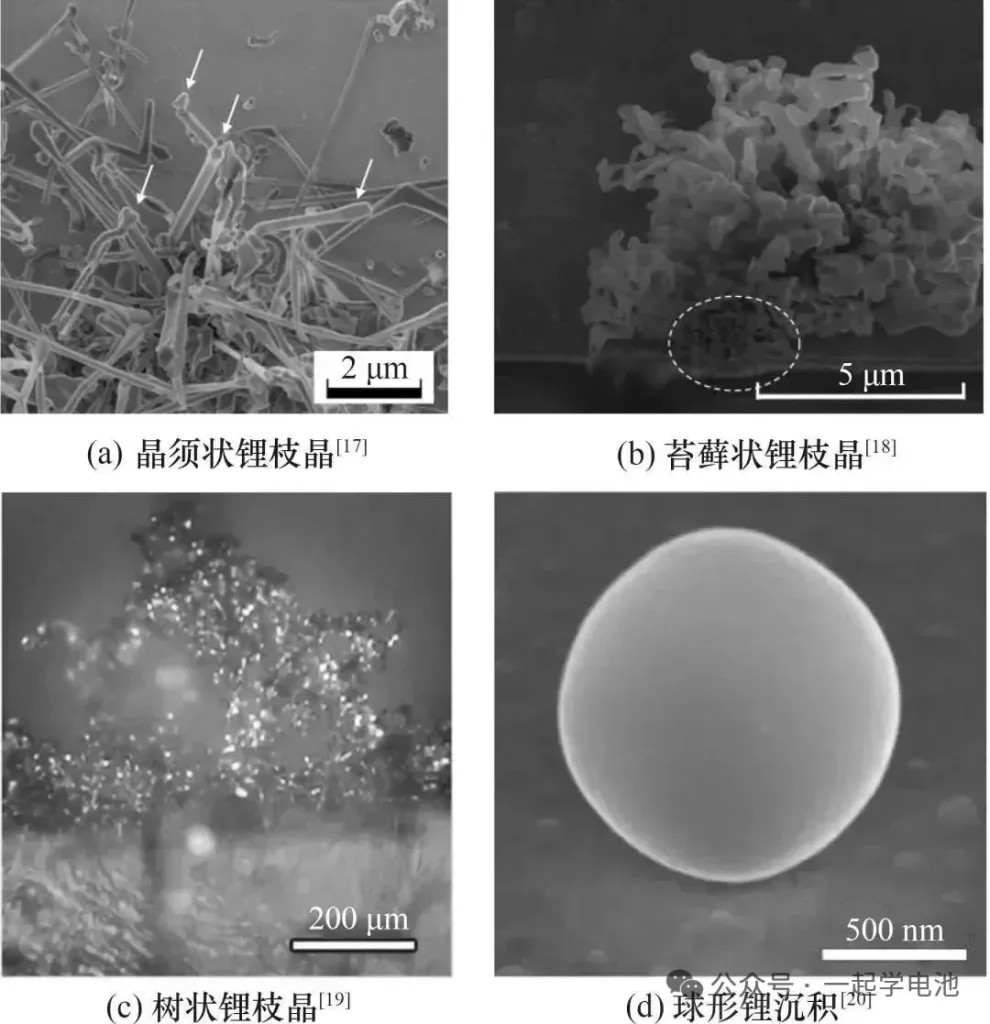
The main causes can be categorized into two major groups: operating conditions and battery states.
1. Operating Conditions (External Factors)
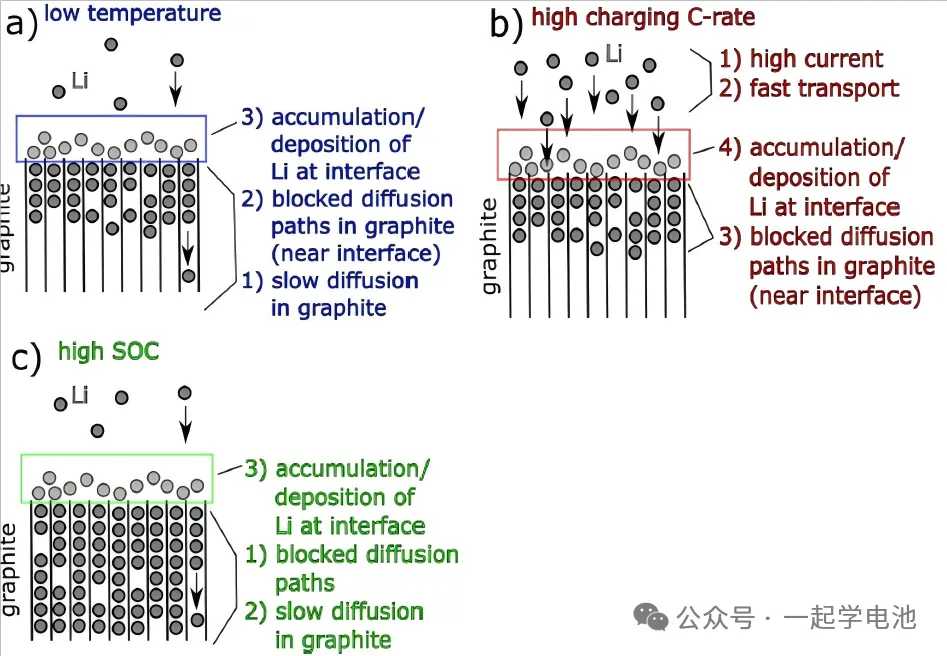
Low-Temperature Charging: This is one of the most common causes. At low temperatures:
The diffusion rate of lithium ions in both the electrolyte and the electrode materials slows down.
The lithium intercalation potential of the graphite anode increases (becomes more positive), making it more likely to approach or drop below the lithium deposition potential (0V vs. Li/Li⁺).
Lithium ions must overcome a higher energy barrier to intercalate into the graphite layers, making the process more difficult.
Result: Lithium ions cannot be intercalated into the graphite layers in time and are forced to reduce and deposit on the surface.

High-Current (Fast Charging): Higher current Neware battery testers
The speed at which lithium ions de-intercalate from the cathode and migrate to the anode surface is too fast.
It takes time for the graphite anode to receive lithium ions and intercalate them into its layered structure (kinetic limitation).
Result: Localized “congestion” forms on the anode surface; the lithium-ion concentration becomes too high, pulling the potential down below the lithium deposition potential.
Overcharging:
The battery is charged beyond its designed upper-limit voltage (e.g., charging to 4.5V instead of 4.2V).
The cathode releases an excessive amount of lithium ions, forcing the anode to receive more lithium while it is already near a fully intercalated state (where the potential is already very low).
Result: The anode potential is forcibly pushed below the lithium deposition potential, leading to lithium plating.

Charging and discharging your batteries
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, over charging, over discharging, or EV driving simulation test, please feel free to contact us.
Maintaining High Voltage/Current at the End of Charging: Even if absolute overcharging is not reached, continuing to charge with high current when the battery is near a full state of charge (high SOC) can easily cause the anode potential to drop too low, leading to lithium plating.
2. Battery State (Internal Factors)
Anode Material Aging or Defects:
Particle Cracking and Pulverization: Graphite particles may crack or pulverize, leading to a reduction in the effective surface area available for lithium intercalation.
Structural Disorder in Graphite: The graphite structure can become disordered, causing the intercalation channels to become obstructed or the intercalation kinetics to deteriorate.

Abnormal SEI Film on Anode Surface:
Excessive or Non-uniform SEI Film: An SEI layer that is too thick or uneven can obstruct the passage of lithium ions, thereby increasing interfacial impedance.
Repeated Rupture and Repair of SEI: Continuous breaking and reforming of the SEI film consumes active lithium and electrolyte.
Insufficient or Poorly Distributed Electrolyte:
Electrolyte Depletion: Battery aging (drying out) or manufacturing defects can lead to localized electrolyte exhaustion.
Limited Ion Transport: Restricted lithium-ion transport results in excessively high localized current densities.
Improper Capacity Ratio Between Positive and Negative Electrodes:
Insufficient Anode Capacity: During the design phase, the negative electrode capacity is insufficient relative to the positive electrode (low N/P ratio).
Rapid Potential Drop: At the end of the charging process, the negative electrode lacks sufficient space for lithium intercalation, causing the potential to be pulled down rapidly.
Internal Short Circuits or Micro-Short Circuits:
These lead to an abnormal localized increase in current density, which induces localized lithium plating.
Manufacturing Defects:
Non-uniform Coating: Variations in electrode coating thickness, the presence of metallic impurities, or non-uniform separator porosity cause localized concentrations of current or ion flux.
II. What Happens When Lithium Plating Occurs?
Lithium plating not only causes a direct loss of active materials but also triggers a series of chain reactions, leading to a significant degradation of battery performance and safety:
1. Accelerated Capacity Fade
Loss of Active Lithium: The deposited metallic lithium reacts with the electrolyte to form a new, thicker SEI film, irreversibly consuming active lithium ions. These ions can no longer participate in the charge-discharge cycles.
Anode Active Material Failure: Lithium metal deposits may cover the graphite surface or clog pores, obstructing the subsequent intercalation and de-intercalation of lithium ions.
Manifestation: Battery capacity drops rapidly during cycling, particularly noticeable after low-temperature or fast-charging cycles.
2. Increased Internal Resistance
Interface Impedance: The precipitated lithium metal and its side-reaction products (the new SEI film) increase the impedance at the electrode/electrolyte interface.
Transport Obstruction: Deposits may hinder the transport of ions and electrons within the electrode.
Manifestation: Voltage changes become more pronounced during charging and discharging (the discharge plateau drops while the charging plateau rises), heat generation increases, and power output decreases.
3. Reduced Coulombic Efficiency
The lithium consumed by plating cannot be fully recovered during discharge (irreversible capacity loss), meaning the electricity input during charging cannot be fully released during discharge.
Manifestation: Charging capacity > Discharge capacity; the charge-discharge efficiency (Discharge Capacity / Charging Capacity) continues to decline.
4. Increased Self-Discharge Rate
The highly active metallic lithium continues to undergo side reactions with the electrolyte, consuming the stored charge.
Manifestation: The voltage drops faster when the battery is at rest, and the charge retention capability deteriorates.
5. Gas Generation (Gassing)
The reaction between lithium metal and the electrolyte produces gases (such as hydrogen and alkanes).
Manifestation: The battery may swell or bulge (most evident in pouch cells), and internal pressure increases.
6. Sharp Increase in Thermal Runaway Risk (Most Dangerous!)
Dendrite Growth: Precipitated lithium metal often grows in the form of dendrites.
Separator Piercing: Sharp lithium dendrites can pierce the separator, causing direct contact between the positive and negative electrodes and triggering an internal short circuit.
Violent Exothermic Reaction: The reaction between lithium metal and the electrolyte is strongly exothermic. An internal short circuit generates massive amounts of heat.
Manifestation: This can lead to severe safety accidents such as fire or explosion. Lithium plating is one of the primary triggers for thermal runaway in lithium-ion batteries.
7. Unique Voltage Characteristics (Basis for Detection)
Abnormal Voltage Plateau at End-of-Charge: When lithium plating occurs, a small plateau or “hump” may appear prematurely in the voltage curve at the end of charging (corresponding to the lithium deposition reaction).
Abnormal Voltage Drop During Rest: In a fully charged state (especially with severe plating), the open-circuit voltage will show a rapid, small-scale drop during the initial rest period due to the continuous reaction of highly active lithium metal. This is distinct from normal, slow self-discharge.
| Category | Causes | Manifestations (Symptoms) |
| External Factors | Low-temperature charging | Rapid capacity fade |
| High current / Fast charging | Increased internal resistance (Heat generation, power drop) | |
| Overcharging | Reduced Coulombic efficiency (Charge more, release less) | |
| High rate at end-of-charge | Increased self-discharge (Fast power loss when idle) | |
| Internal Factors | Anode aging/defects (Reduced surface area, difficult intercalation) | Gassing and bulging |
| Abnormal SEI film (Increased resistance) | Sharp increase in thermal runaway risk (Danger! Fire/Explosion) | |
| Insufficient/Uneven electrolyte | Abnormal end-of-charge voltage plateau / Rapid voltage drop during rest | |
| Low N/P ratio (Insufficient anode capacity) | ||
| Internal micro-short circuits / Manufacturing defects |
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
