Optimizing Zinc Batteries: From Anode Materials to Performance Data Analysis
Source: WeChat Official Account “Learn Batteries Together” 来源于微信公众号 一起学电池
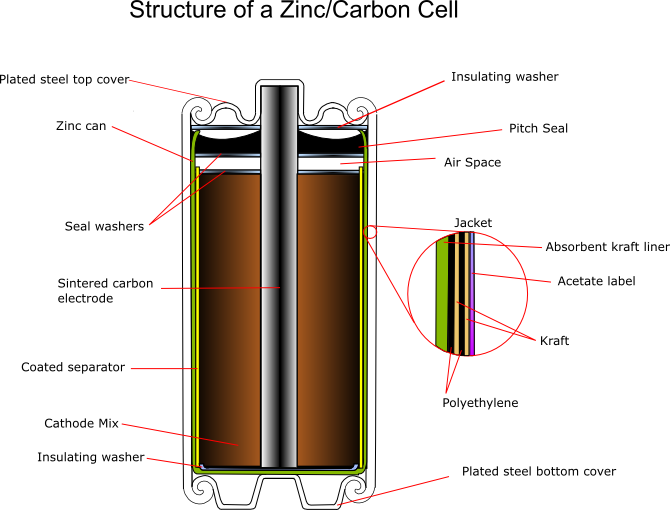
Research on Zinc Batteries Anodes
Zinc Batteries Anodes:
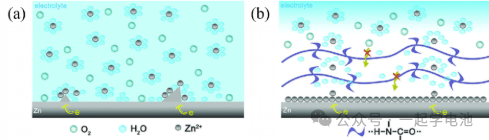
The construction of an artificial interface layer enables uniform Zn²+ deposition by providing spatial shielding and guiding homogeneous ionic diffusion. Spatial shielding utilizes the interface layer to physically block dendrite growth directly. Guiding uniform Zn²+ diffusion is achieved through mechanisms such as electrostatic interactions, chemical adsorption, and the construction of ion transport channels (ion tunnels).
Artificial Interface Layer:
Organic materials—specifically integrated thin films characterized by highly reversible shape changes and covalent bond cross-linking—serve as excellent candidates for artificial interface layers. The figure illustrates an all-in-one polyamide (PA) membrane fabricated via the solution-casting-drying method. Leveraging its unique hydrogen-bonding network and strong coordination capability with metal ions, this membrane coordinates Zn²+ migration through uniform nucleation, effectively regulating zinc deposition behavior.
Constructing Ion Tunnels:
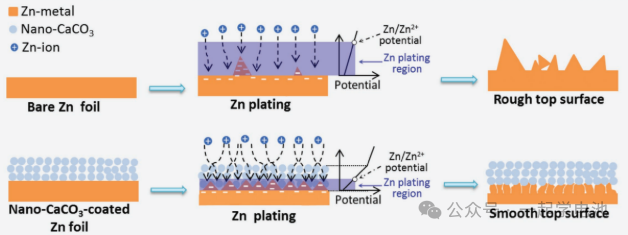
CaCO₃ possesses a well-developed nanoscale porous structure. Utilizing it as a coating for zinc battery anodes facilitates the selective control of Zn2+ deposition sites.
Constructing Ion Channels/Tunnels:
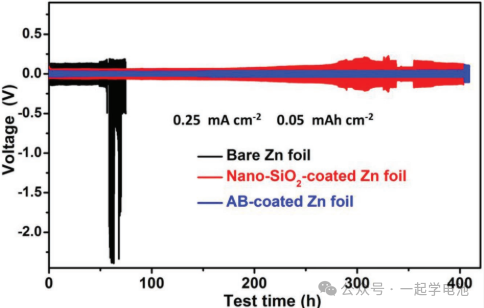
Porous nano-SiO₂ and AB layers were further deposited on zinc foils, and their galvanostatic cycling stability was evaluated. Experimental results demonstrate that nano-CaCO₃, nano-SiO₂, and AB porous coatings can effectively extend the cycling life of Zn|ZnSO₄+MnSO₄|Zn symmetric batteries by guiding uniform and site-selective zinc plating.
Alloy Coating:
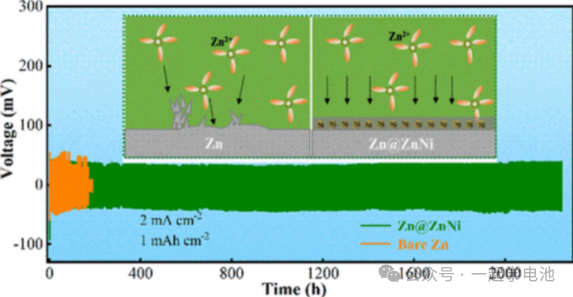
A ZnNi alloy coating was electrodeposited on the surface of Zn foil. First-principles calculations indicate that the binding affinity between the ZnNi alloy and Zn ions is stronger than that of bare Zn metal. This suggests that Zn nuclei preferentially form around the ZnNi alloy rather than continuing to grow on the initial surface of the Zn electrode. Consequently, the fabricated symmetric battery achieved a cycling life exceeding 2,200 hours at a current density of 2 mA·cm⁻².
Research on Zinc Batteries Electrolytes
Zinc Batteries Electrolytes:
Alkaline electrolytes cause severe zinc dendrite issues and the formation of $ZnO$ by-products, while acidic electrolytes lead to the corrosion of the zinc anode and current collectors. Consequently, neutral or mildly acidic electrolytes are the most commonly used in aqueous zinc-ion batteries. The zinc salts under investigation primarily include Zn(CF₃SO₃)2、ZnSO4、Zn(NO₃)2、Zn(CH₃COO)2、ZnF₂、Zn(CIO4)2、and ZnCl₂.
Electrolyte design for aqueous zinc-ion batteries aims to suppress dendrite formation and enhance low-temperature performance. The primary strategies for improvement include:
Constructing “Water-in-Salt” (WiS) electrolytes.
Incorporating organic or inorganic additives.
Fabricating hydrogel electrolytes.
Ethylene Glycol (EG) as an Electrolyte Additive:
By leveraging the strong covalent bonding between EG and water molecules, the solvation effect of $Zn^{2+}$ is weakened. Furthermore, the addition of EG lowers the freezing point of the electrolyte and enhances its ionic conductivity under low-temperature conditions. This allows the fabricated zinc-ion batteries to undergo charge-discharge cycles with high capacity retention even at -40°C.
In addition to EG, adding solvents such as Dimethylformamide (DMF), Acetonitrile (ACN), and Acetamide similarly provides the dual benefits of lowering the freezing point and promoting uniform zinc ion deposition, potentially enabling stable operation at temperatures as low as -70°C.
Structural Optimization of Hybrid Electrolytes via MD Simulation and DFT Calculations:
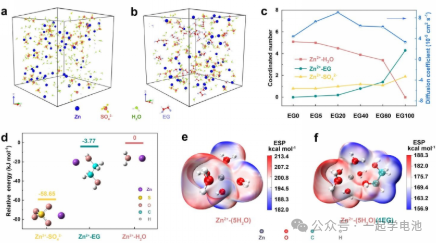
The structure of the hybrid electrolyte was optimized through Molecular Dynamics (MD) simulations and Density Functional Theory (DFT) calculations.
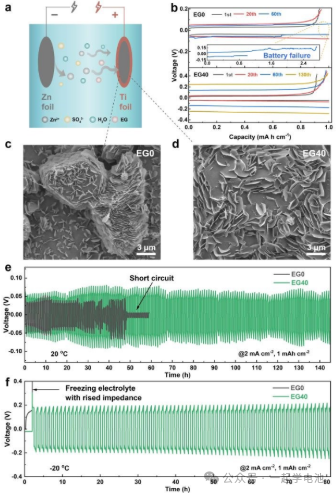
(a) and (b): MD simulation snapshots of EG0 and EG40 electrolyte systems.
(c): MD simulation results calculating the coordination numbers of H₂O, EG, and SO₄2 around Zn²+, as well as the diffusion coefficients of Zn²+ in different electrolyte environments.
(d): Relative binding energies of various Zn²+ species obtained through DFT calculations.
(e) and (f): Electrostatic Potential (ESP) maps of the original Zn²+-H₂O system and the system with EG additives.
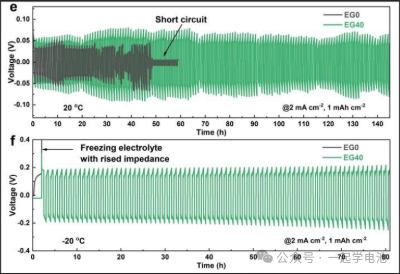
Electrochemical testing and physical characterization of EG0 and EG40 were conducted using zinc symmetric batteries. (a) Schematic diagram of the Ti | Zn battery. (b) Voltage profiles of the Ti | Zn battery at 2 mA/cm². SEM images of zinc deposition on Ti foil after plating a capacity of 2 mAh/cm² of Zn in Ti | Zn batteries using (c) EG0 and (d) EG40. Cycling stability of the Zn batteries at (e) 20°C and (f) -20°C.
“Water-in-Salt” (WiS) Electrolyte:
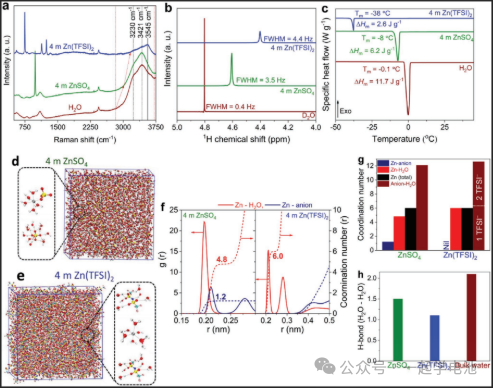
A zinc bis(trifluoromethylsulfonyl)imide (Zn(TFSI)2) aqueous solution serves as a stable electrolyte. Compared to traditional ZnSO4, the Zn(TFSI)2 electrolyte reduces the Zn2+ solvation effect, demonstrating enhanced ion transport characteristics, superior electrochemical compatibility, and improved battery performance.
Full cells assembled with this electrolyte provided significant capacities of 324 and 98 mAh/g at 1 C and 450 C, respectively, alongside exceptional cyclability. Over 48,000 cycles at 30 C, the capacity decay rate was merely 0.00035% per cycle. Notably, the highly concentrated salt system lowers the freezing point, enabling the zinc-ion battery to operate under low-temperature conditions. Even at an extreme temperature of -35°C, the battery maintains a high capacity of 178 mAh/g.
Physicochemical properties of 4 M ZnSO4 and Zn(TFSI)2 aqueous solutions. Vertically offset (a) Raman spectra, (b) 1H NMR spectra, and (c) Differential Scanning Calorimetry (DSC) curves. Simulation snapshots of (d) 4 M ZnSO4 and (e) Zn(TFSI)2 electrolytes during the DFT-MD simulation process.
Preparation of Hydrogel Electrolyte (Semi-Solid Electrolyte):
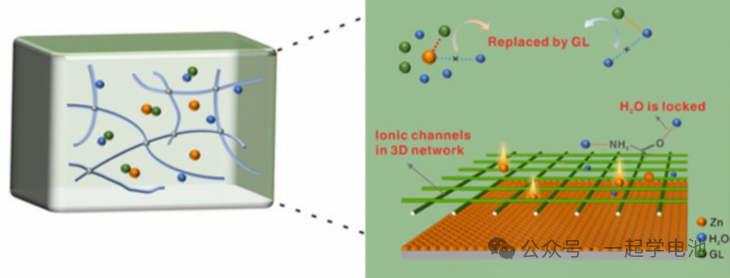
A gel electrolyte is a network structure composed of water and a polymer matrix. It is a promising aqueous electrolyte that can both suppress side reactions and prevent leakage. Flexible pouch cells prepared using this hydrogel as a base exhibit excellent mechanical stability, maintaining stable voltage and capacity under various bending angles. They can be utilized for the research and fabrication of wearable batteries.
The figure illustrates a hydrogel with a three-dimensional cross-linked network structure. The abundant ion channels within the gel facilitate the directional migration of Zn2+, thereby regulating Zn2+ deposition. The glycerol (GL) in the gel and the hydrophilic groups on the gel network exhibit higher binding energy with water molecules (i.e., they form connections with water molecules more easily). This disrupts the hydrogen bonding structure between water molecules, significantly lowering the freezing point of the electrolyte and enhancing the anti-freezing properties of the gel.
Ultimately, this achieves a wide operating temperature range from -20 to 60°C, as well as a capacity of 185 mAh/g after 10,000 cycles at 5 A/g.
Data Interpretation
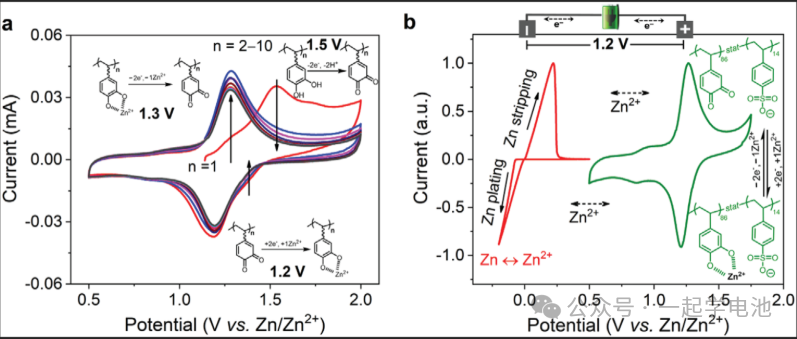
Cyclic Voltammetry (CV):
The CV curves for aqueous zinc-ion batteries are relatively simple and clear. By observing the voltages where sudden current changes occur, the shifts at the upper and lower voltage limits during charging/discharging can be attributed to the Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER). The redox peaks in the middle represent the oxidation or reduction reactions of Zn2+ on the electrode, which generate current signals that form peaks between potential and current. Based on the position, shape, and magnitude of these redox peaks, one can investigate the nature of electrode reactions, reaction mechanisms, reaction rates, and kinetic parameters of the electrode process.
Symmetric Cell Cycling:
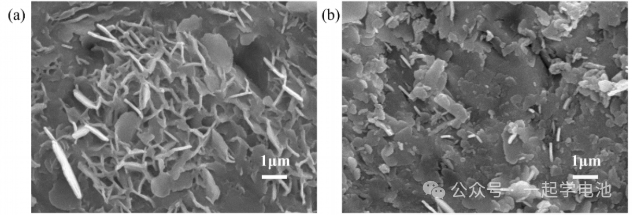
Figures (a-b) show the surface SEM images of Zn-Zn symmetric cells after completion of cycling performance tests. Figure (a) exhibits obvious zinc dendrites, while Figure (b) reveals that the zinc foil electrode has undergone severe corrosion (dissolution).
The figure below shows the cycling performance curves of the Zn-Zn symmetric cells. Galvanostatic charge-discharge tests were conducted on symmetric cells using different electrolytes at the same current density over time to investigate the impact of the electrolytes on the zinc foil electrodes.
Neware charge and discharge testers
YouTube: New Zinc Battery Lasts 12 Million Miles — And It’s Already Been Tested
Related News:
- How do aqueous zinc-ion batteries relate to traditional Zn//MnO₂ electrochemical systems? 2026 post
- A Bifunctional Separator with Gradient Distribution of MCM-41 Zeolite for High-Performance Aqueous Zinc-ion Batteries
- Zn//MnO2 battery from Primary to Rechargeable: A Timeline of Key Breakthroughs in Aqueous Zinc-Ion Batteries (AZIBs) 2026 post
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-2