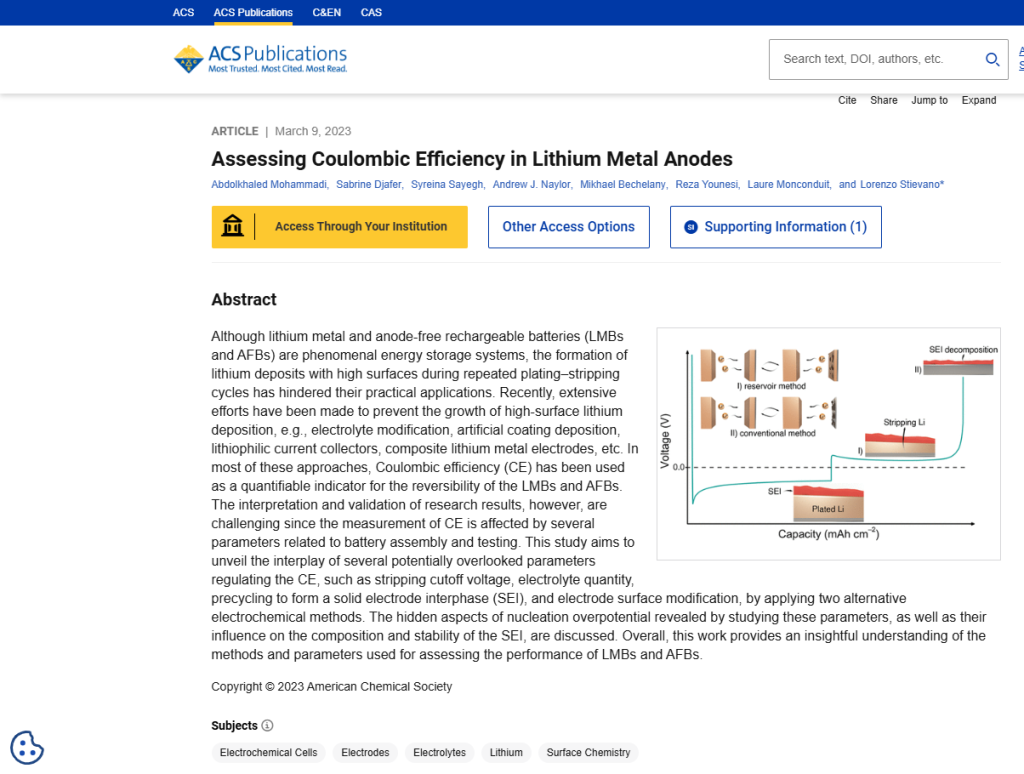
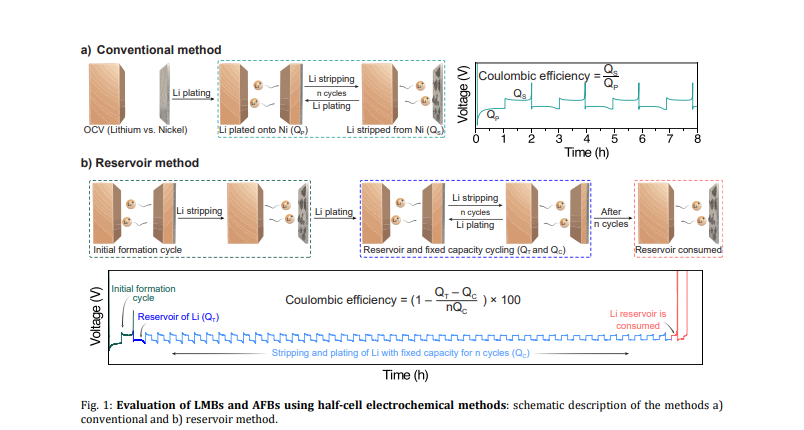
CONCLUSIONS
Two different electrochemical methods were utilized in this study to highlight the effect of different experimental factors, which are often neglected in many studies, on the measurement of CE and cycling performance of LMBs/AFBs. Hidden and inconspicuous electrochemical and cell parameters can substantially affect the performance of the benchmark system. As a result of the findings in this paper, several key points were clarified:
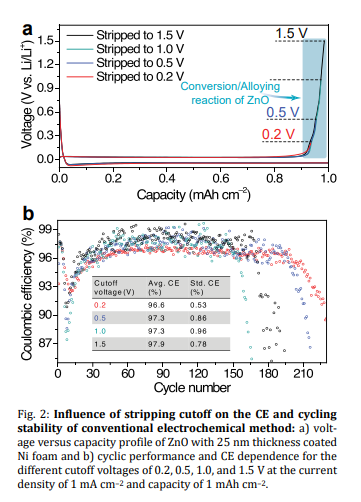
Cutoff: Despite the fact that the average CE decreases by lowering the upper cutoff potential in the conventional electrochemical method, the cycle life can be extended. In other words, increased high cutoff potentials lead to reduced lifespan due to the further decomposition of electrolytes and irreversible electrochemical reactions. Accordingly, the lower cutoff voltage is recommended for lithium plating and stripping tests.
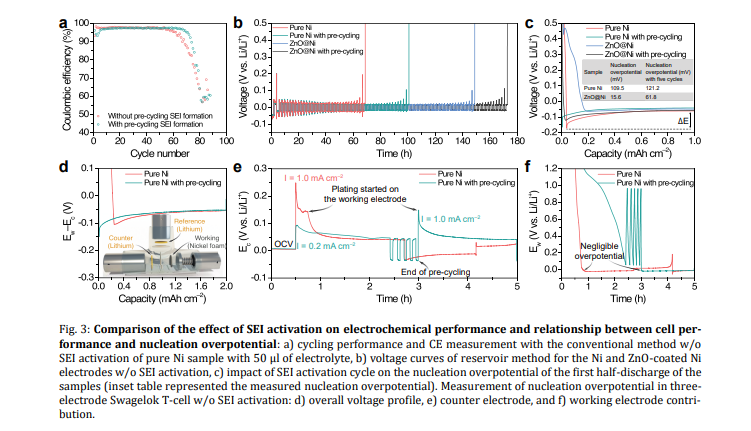
Pre-cycling for initial SEI formation: Galvanostatic precycling to form and stabilize SEI is here shown to not affect the cycling performance and CE measurements for the conventional electrochemical method. Most probably, the stabilized SEI can be easily changed and damaged by the complete stripping of lithium and the continued plating of Li. However, in the reservoir method, stabilizing the SEI via pre-cycling before lithium deposition improved CE values. Due to the initial SEI formation, the nucleation overpotential of the electrode increases; this allows the unveiling of the hidden aspects of the nucleation overpotential of the batteries. Use Neware battery cyclers for Pre-cycling
Nucleation overpotential: The results from three-electrode cells indicate that the energy barrier for stripping or extracting lithium from the lithium metal counter electrode is significantly higher than the energy barrier for forming nucleation sites on the working electrode when the cell starts cycling. This also explains why the pre-cycling to form initial SEI induced a higher nucleation overpotential.
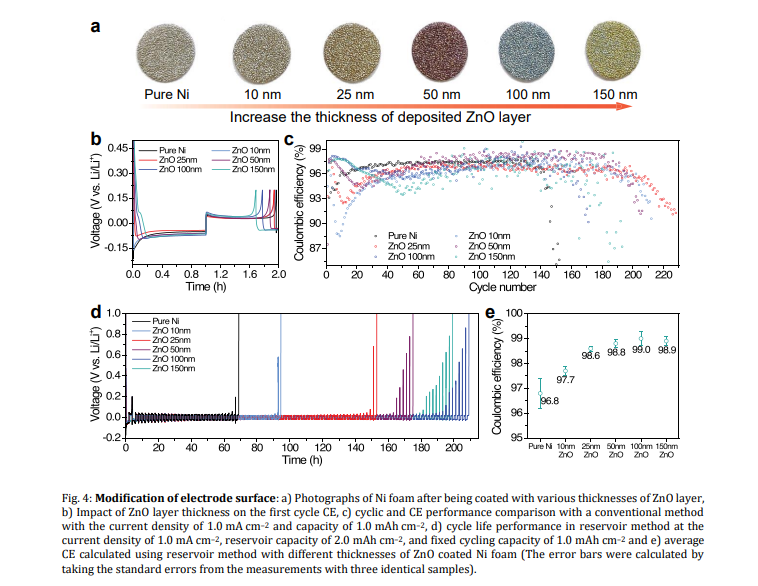
Modification of electrode surface: The optimal thickness of ZnO coating on the Ni foam current collector was shown to be below 50 nm when using the conventional method, whereas increasing the thickness improves the performance in the reservoir method. The results indicate that the lower thickness of ZnO would be more suitable for use in AFBs with a limited source of lithium in the cathode electrode, whereas a higher thickness of ZnO would be more beneficial for infusing molten lithium to use as an anode in the LMBs. In general, it is challenging to determine the effective optimal thickness of coatings in modified materials.
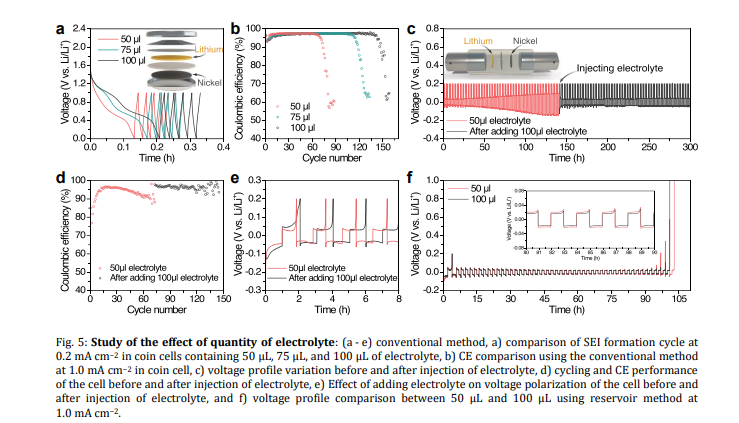
Quantity of electrolyte: In the conventional electrochemical method, the mechanism of cell failure is electrolyte depletion rather than dendrite formation, and the cycle life of a battery is directly proportional to its electrolyte volume. In contrast, the reservoir method shows that the failure is independent of the amount of electrolyte. Furthermore, XPS analysis reveals that the evolution of SEI during lithium plating and the entire stripping of lithium for each cycle leads to electrolyte depletion in the conventional electrochemical method.
SEI in AFBs: The results for AFBs show similar that the cycled life could be improved by keeping some metallic lithium on the anode electrode, resulting in higher stability of the SEI and thus higher CE values.
We believe that by gathering this type of information, researchers may be able to gain a deeper understanding of the efficiency of different parameters but also of different analytical methods in the thorough understanding of lithium plating and stripping processes.
Article title: Assessing Coulombic Efficiency in Lithium Metal Anodes https://doi.org/10.1021/acs.chemmater.2c03518
Source: WeChat Official Account “Brother Radish” 萝卜大师兄