Silicon vs. Graphite: The Micro-War Defining the Future of Battery Performance
Under the microscope, silicon and graphite particles act like two athletes with starkly different temperaments, collaborating yet competing fiercely on the charging and discharging stage; the fate of the battery hinges on the outcome of this microscopic gambit.
The composite electrode is formed by mechanically blending nano-silicon particles with micron-sized graphite particles, yielding electrochemical performance that is significantly superior to that of single-material batteries.
Silicon boasts a staggering theoretical capacity of 3,579 mAh/g—nearly ten times that of traditional graphite anodes—positioning it as a pivotal material for achieving high-energy-density batteries. By optimizing the competitive interplay between silicon and graphite, it is possible to engineer next-generation lithium-ion batteries that offer both superior energy density and extended cycle life.
01 The Genesis of Co-opetition Silicon vs. Graphite
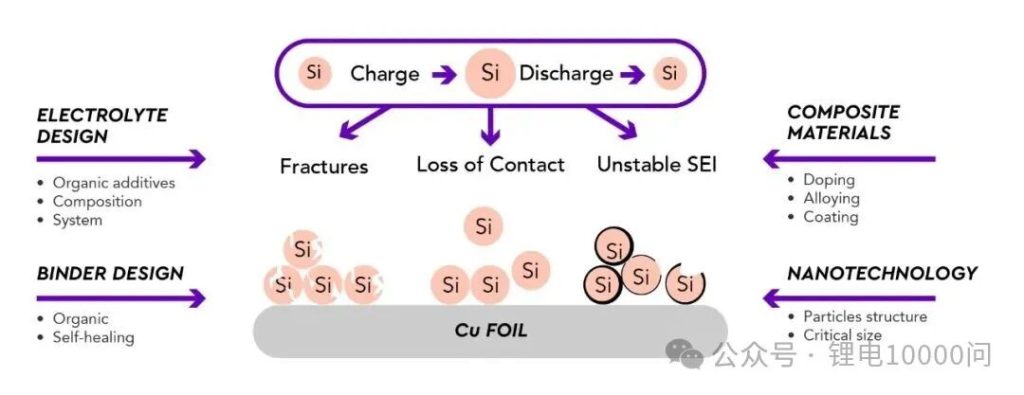
The commercialization of silicon-based anodes has long been hindered by a fundamental contradiction. During the lithiation and delithiation processes, silicon undergoes an alloying reaction with lithium, accompanied by a staggering 300%–400% volume expansion and contraction.
This violent “breathing effect” leads to the pulverization of active particles and the catastrophic failure of the conductive network. Furthermore, it continuously ruptures and regenerates the Solid Electrolyte Interphase (SEI) layer, consuming active lithium and accelerating capacity fade.
Graphite exhibits a completely different “persona”: its lithium storage mechanism relies on the intercalation and de-intercalation of lithium ions between its layers, resulting in a modest volume change (approx. 10%) and exceptional structural stability. However, it faces a low theoretical capacity ceiling of only 372 mAh/g.
Blending silicon with graphite is regarded as a “golden combination” that leverages the strengths of both materials. The stable framework of graphite serves as a buffer against silicon’s volume expansion, while silicon significantly boosts the overall capacity of the electrode.
However, it is far from a simple physical superposition. When silicon and graphite coexist, they engage in a silent “resource competition” at the microscopic level for limited lithium ions, electronic pathways, and physical space.
A significant discrepancy exists between their lithiation potential plateaus. Graphite lithiation primarily occurs below 0.2 V (vs. $Li^+/Li$), whereas the lithiation reaction for silicon begins at approximately 0.3 V – 0.4 V and continues down to nearly 0 V.
Consequently, during the charging process, lithium ions “preferentially” react with silicon. This potential difference is the root of the competition between the two materials, dictating that they cannot operate synchronously or uniformly.
02 Competitive Mechanisms Silicon vs. Graphite
Deep within the electrode, the competition between silicon and graphite is a complex gambit involving reaction sequences, kinetics, and stress fields. Recent research, utilizing advanced in-situ synchrotron X-ray diffraction (XRD), has revealed the component-specific dynamic processes during charge and discharge.
In the early stages of charging (lithiation), when the voltage is above approximately 200 mV, amorphous silicon begins to lithiate preferentially due to its higher reaction potential. At this point, graphite remains largely in a “standby” state.
When the voltage drops below 200 mV, the competition intensifies. Amorphous silicon, graphite, and potentially crystalline silicon begin to compete fiercely for the limited supply of lithium ions.
This competition directly alters the conventional lithiation stages of graphite. Research indicates that the presence of silicon shifts the transition potentials of graphite intercalation compounds (GICs). Consequently, at the same voltage level, the state of charge (SOC) of graphite within a composite electrode is significantly lower than that in a pure graphite electrode.
In other words, silicon’s “line-cutting” disrupts the “dining rhythm” of graphite. More critically, the two materials remain out of sync during the discharge (delithiation) process. The delithiation sequence follows a pattern where graphite reacts first, followed by the amorphous lithium-silicide phases.
This divergence between lithiation and delithiation pathways leads to non-uniform lithium distribution and hysteresis within the electrode, laying the groundwork for performance degradation.
Beyond kinetic competition, there is an even more intense mechanical conflict. Upon lithiation, silicon particles expand drastically, “crowding out” the surrounding space. Electrochemical-mechanical coupled models reveal that in a uniformly mixed electrode, the preferential lithiation and expansion of silicon significantly suppress the degree of lithiation in adjacent graphite regions.
The expansion of silicon constricts the pore space required by graphite, hindering electrolyte wetting and lithium-ion transport. This triggers a “lithium crosstalk” phenomenon: while some graphite regions are unable to store lithium effectively, certain silicon particles may fail due to stress concentration.
03 The Impact of Composition Ratios Silicon vs. Graphite
The silicon-to-graphite ratio serves as the “control knob” for regulating this competition. Varying this ratio leads to dramatic shifts in the dominant failure mechanisms and overall performance.
Low-silicon systems (e.g., 5%–10%) currently represent the mainstream for commercialization. In these systems, graphite acts as the continuous phase, while silicon serves as a “decorative” addition for capacity enhancement. The graphite network is robust enough to buffer silicon’s expansion, meaning competitive conflicts are not prominent, and the battery maintains good cycling stability, albeit with limited gains in energy density.
As silicon content increases to medium and high levels (e.g., above 30%), competitive effects become pronounced, and new bottlenecks emerge.
Studies on electrodes with 30% and 70% silicon content revealed that in 30% silicon electrodes, the amorphization process of crystalline silicon—occurring at low voltages ($< 110\ mV$)—faces significant kinetic hindrance under higher discharge rates (above C/5).
This implies that under fast-charging conditions, a portion of the silicon cannot react completely, preventing the full utilization of its capacity. In electrodes with high silicon content (70% Si), the situation becomes even more intricate: silicon expansion becomes the absolute dominant factor, severely restricting lithium diffusion within the graphite phase. Simultaneously, it promotes the formation of crystalline alloy phases such as Li₁₅Si₄, which is highly detrimental to cycling stability.
Interestingly, this competitive relationship is also sensitive to temperature. Research on solid-state battery (SSB) systems indicates that at room temperature, increasing silicon content generally helps improve capacity retention and rate performance, as silicon contributes the bulk of the capacity.
However, at elevated temperatures (e.g., 60 °C), the trend reverses: high silicon content may instead lead to faster performance degradation. This reveals that other factors, such as side reactions and interfacial stability, begin to intervene in the competition, further complicating the landscape.
If you need to test your batteries at 60℃ or higher temp, please click here: Neware environmental chamber
04 Spatial Breakthroughs Silicon vs. Graphite
Since competition is inevitable, how can we steer it toward a favorable outcome? Altering the spatial distribution of silicon and graphite within the electrode represents a revolutionary “breakthrough” strategy.
Traditional uniform blending forces silicon and graphite into “hand-to-hand combat,” exacerbating crosstalk and mechanical interference. Cutting-edge research has proposed subversive “gradient designs” or “layered architectures.”
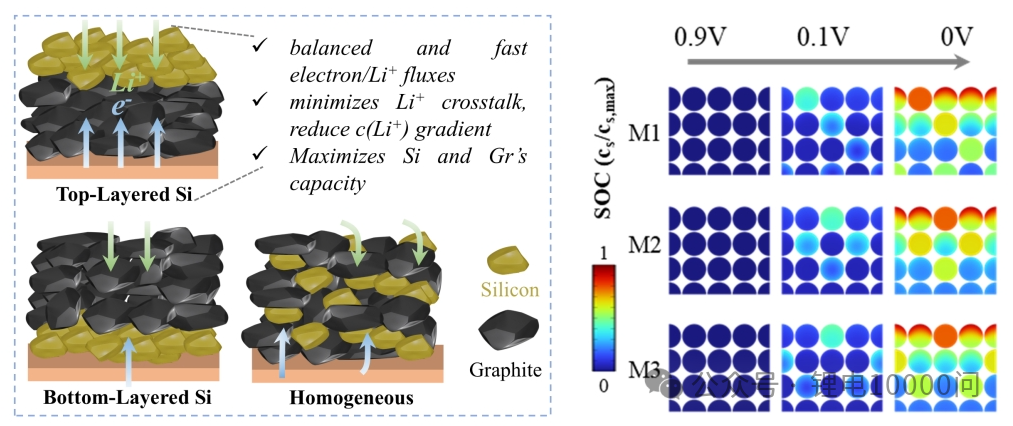
A research team from Fujian Normal University has confirmed, through both modeling and experimentation, that a strategic arrangement—placing silicon particles primarily in the surface layer (near the separator) and graphite in the bottom layer (near the current collector)—can produce remarkable results.
In this configuration, lithium ions entering from the separator side first encounter the silicon layer. Due to silicon’s high reaction potential, it undergoes preferential and rapid lithiation. At this stage, the underlying graphite layer has not yet begun to react, providing a spatial buffer for silicon’s expansion upward (toward the separator). This prevents the two materials from crushing each other.
Subsequently, lithium ions diffuse further into the graphite layer to initiate its lithiation. This design achieves the “decoupling” of silicon and graphite in both time and space, effectively suppressing crosstalk, reducing polarization, and enhancing overall kinetics.
A team from Beijing University of Chemical Technology validated the benefits of spatial design from another perspective. Their gradient silicon/graphite anode leverages the lower desolvation energy and faster ion transport capabilities of the surface silicon to preferentially accept lithium during fast charging. This can even induce the “self-dissolution” of deposited lithium metal, thereby significantly inhibiting lithium dendrite growth. As a result, the full cell maintained a high capacity retention of 97.9% after 300 cycles under 4C fast-charging conditions.
05 Material Synergy Silicon vs. Graphite
Beyond macro-electrode architecture, constructing more integrated “silicon-carbon composites” at the microscopic level is another core strategy for mitigating competition—yielding results far superior to simple physical blending.
Composite strategies strive for a chemical integration where the materials are inextricably intertwined. This is achieved through techniques such as Chemical Vapor Deposition (CVD), ball-milling, or sol-gel methods, which enable the direct coating of carbon layers onto silicon nanoparticles or their embedding within a carbon matrix.
A “sandwich-like graphene/silicon/graphene” composite developed by a team from the East China University of Science and Technology serves as an excellent paradigm. Silicon nanoparticles are encapsulated between two layers of reduced graphene oxide (rGO). The mechanical flexibility and interlaminar voids of graphene provide a dedicated buffering space for silicon’s expansion, while its highly conductive network ensures rapid electron transport.
This architecture realizes a “gradient design on a microscopic scale,” effectively transforming competition into synergy. The carbon layer acts as a stable conductive scaffold and buffering matrix, while the silicon, serving as the high-capacity core, remains both protected and interconnected.
Research demonstrates that this composite electrode maintains a high capacity retention of 95.3% after 200 cycles at a current density of 0.5 A/g. In contrast, in electrodes formed by simple physical blending, silicon and graphite particles tend to undergo agglomeration, leading to poor electrical contact and obstructed lithium-ion transport pathways, which results in significantly inferior performance.
Industry leaders have also taken action. BTR, a global leader in anode materials, recently unveiled its innovative “BTR S+i Graphite” solution. This approach focuses on innovation at the graphite end by designing specialized graphite materials that are better matched to silicon’s characteristics. By doing so, it reduces “lithium crosstalk” and improves dispersion, achieving a “1+1 > 2” synergistic effect even at high silicon ratios.
The competition between silicon and graphite in composite electrodes has been effectively channeled through innovative gradient electrode designs. By meticulously placing silicon in the top layer near the separator, it reacts preferentially as lithium ions flow in; its expansion is then directed toward the exterior of the electrode, preserving internal space for the subsequent lithiation of the underlying graphite layer. In this ordered microscopic world, battery energy density and cycle life have found a new point of equilibrium.
Source:
1、Decoupling the impact of silicon-graphite spatial configuration on Li-storage performance via electrochemical-mechanical coupling model;
2、Fast-charging graphite-based anode enabled by gradient silicon: from mechanism revelation to electrode design;
3、Spatial-Dependent Coupling of Electrochemistry, Mass Transport, and Stress in Silicon-Graphite Composite Electrodes for Lithium-Ion Batteries;
4、A sandwich rGO/Si/rGO material as a high-performance anode material for lithium-ion batteries
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
