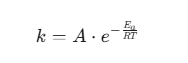Temperature has a significant impact on battery performance, particularly in lithium-ion batteries, which are widely used in various applications due to their high energy density and stability. Here’s a detailed overview of the effects of temperature on batteries.
Performance at High Temperatures
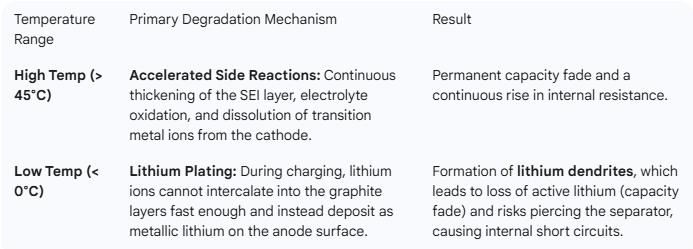
High temperatures, especially above 35°C (95°F), can accelerate chemical reactions within the battery, leading to faster degradation and reduced lifespan .Overheating can cause thermal runaway, a dangerous condition where the battery can catch fire or explode . Prolonged exposure to high temperatures also increases safety risks and can lead to device failure .
Performance at Low Temperatures
Low temperatures, particularly below 0°C, impair charging efficiency as the internal resistance of the battery increases, and the maximum voltage is reached earlier . This can result in longer charging times and reduced capacity. During discharge, low temperatures increase internal resistance, reducing power output and capacity . Operating at low temperatures can also weaken acceleration and reduce the runtime of devices .
Storage Temperatures
For lithium batteries, the recommended storage temperature range is -20°C to 25°C (-4°F to 77°F) . Storing batteries outside this range can accelerate aging or cause irreversible damage . In extreme climates, insulation or heated storage areas can prevent freezing, while cool, shaded areas or climate-controlled environments are recommended for hot climates .
Charging at Extreme Temperatures
Charging lithium batteries at low temperatures decreases efficiency, leading to slower charging times and reduced capacity . High temperatures during charging can cause overheating and thermal runaway, posing safety hazards . The optimal charging temperature range is 0°C to 45°C (32°F to 113°F) .
Discharging at Extreme Temperatures
Discharging at extreme temperatures also affects performance and lifespan. Low temperatures increase internal resistance, while high temperatures can accelerate chemical reactions, leading to faster degradation and capacity loss . It’s best to avoid discharging below -20°C (-4°F) or above 60°C (140°F) .
Thermal Management Systems
To mitigate the effects of temperature on batteries, thermal management systems are crucial. These systems help maintain optimal battery temperature, especially in electric vehicles, where temperature effects can significantly impact performance and range .
Temperature’s impact on battery performance can be described as a “double-edged sword.” From an electrochemical perspective, temperature directly dictates the thermodynamic equilibrium (potential) and kinetic rates (ion migration and reaction) within the battery.
Here is a breakdown of how temperature affects battery performance across several core dimensions:
1. Kinetics and Internal Resistance
The ion transport and reaction rates inside a battery follow the Arrhenius Law:
In summary, temperature plays a critical role in the performance, safety, and lifespan of batteries. Managing battery temperature effectively is essential to ensure optimal operation and to prevent damage due to extreme temperatures.
Where k is the reaction rate, and T is the absolute temperature.
- High Temperature (within reasonable limits): This increases the diffusion rate of ions in the electrolyte and reduces electrolyte viscosity, thereby lowering the internal resistance. Consequently, batteries at higher temperatures often exhibit higher discharge power and better rate performance.
- Low Temperature: The electrolyte becomes viscous or may even partially crystallize, causing a surge in ion migration resistance. This leads to a significant voltage drop (IR drop), causing the battery to hit its cut-off voltage prematurely during high-current discharge, which results in a notable decrease in available capacity.
2. Capacity and Energy
Cold Environments: Capacity is not permanently “lost”; rather, it becomes inaccessible because the kinetics are hindered. The active materials cannot be fully utilized within the discharge window.
Hot Environments: Material activity is higher, often allowing the battery to release slightly more capacity than at room temperature. However, this gain is short-term and comes at the expense of the battery’s overall lifespan.
3. Lifespan and Degradation
This is where temperature has its most profound and lasting impact.
4. Safety and Thermal Runaway
When the temperature rises above critical thresholds, the battery enters a series of irreversible, self-heating chain reactions:
- 70°C – 90°C: The SEI layer begins to decompose, exposing the unstable anode to the electrolyte and triggering exothermic reactions. Neware high and low temp chamber( -70/-40/-20~150℃)
- 130°C – 150°C: The separator melts, leading to large-scale internal short circuits.
- 200°C+: The cathode material decomposes and releases oxygen, leading to violent combustion of the electrolyte—a phenomenon known as Thermal Runaway.
5. The Battery “Comfort Zone”
Like humans, batteries have a preferred operating temperature range:
• Optimal Operating Temp: 15°C to 35°C. Neware 0~60℃ chamber
• Optimal Charging Temp: 10°C to 30°C (charging at high currents in low temperatures is strictly prohibited).