Why does an unstable SEI film always form during battery testing?
SEI film, short for Solid electrolyte interphase, is an important concept in lithium-ion batteries. It is a composite film formed on the surface of the battery’s negative electrode material, and has the following characteristics:
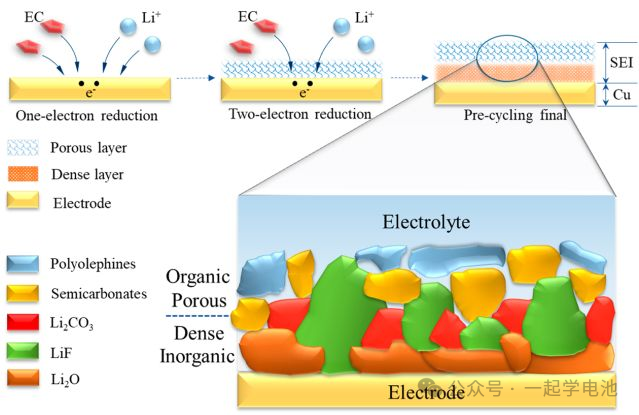
Electronic Insulator: The SEI film prevents electrons from transferring directly from the electrode to the electrolyte, thereby avoiding direct reactions between the electrode and the electrolyte.
Ionic Conductor: Although the SEI film is insulating to electrons, it allows lithium ions (Li+) to pass through, enabling lithium ions to move between electrodes during the battery’s charging and discharging processes.
Protective Layer: The SEI film acts as a protective layer between the electrode material and the electrolyte, preventing solvents and lithium ions in the electrolyte from directly contacting the electrode material, thus protecting the electrode material from corrosion.
Passivation Layer: During the initial charge and discharge cycles of the battery, the surface of the electrode material reacts with the electrolyte to form the SEI film; this process is known as passivation.
Impact on Battery Performance: The uniformity, stability, thickness, and composition of the SEI film have a significant impact on battery performance, including capacity, cycle life, and safety.
In summary, the presence of the SEI film is crucial for improving the performance and safety of lithium-ion batteries. The continuous growth of the SEI film caused by the instability of the anode-electrolyte interface has several negative impacts, specifically including:
Increasing Irreversible Capacity Loss: Instability of the SEI film leads to more lithium ions and electrolyte being consumed in the formation and maintenance of the SEI, resulting in a reduction of cyclable lithium ions and thus lowering the battery’s capacity. During each charge-discharge cycle, more lithium is trapped in the SEI film and cannot participate in reactions.
Increasing Internal Resistance: The continuous thickening of the SEI film increases the internal resistance of the battery and hinders the conduction of lithium ions. High internal resistance not only reduces the energy efficiency of the battery but also leads to a significant decline in performance during high-rate discharge.
Reducing Charge-Discharge Efficiency: Due to the instability of the SEI film, more lithium ions are permanently lost within the SEI film during the initial charge-discharge process, thereby reducing the battery’s coulombic efficiency.
Shortening Battery Cycle Life: Capacity decay is caused by the continuous growth of the SEI film and the constant consumption of lithium ions. As the SEI film grows, the active surface area of the anode material gradually decreases, which limits the battery’s cycle life. With an increasing number of cycles, the thickness of the SEI film increases, further accelerating capacity fading.
Non-uniformity of Battery Performance: Non-uniform growth of the SEI film leads to an uneven current density distribution on the electrode surface, resulting in inconsistent battery performance. This non-uniformity may cause localized overheating or over-discharge, leading to internal short circuits or thermal runaway, which affects the safety and stability of the battery.
Affecting High-Temperature Performance: Under high-temperature conditions, the SEI film may restructure and form a porous structure, allowing the electrolyte to come into further contact with the electrode and continue the reduction process, which impacts the high-temperature performance and safety of the battery.
Formation of Lithium Dendrites: An unstable SEI film may lead to the rapid deposition of lithium ions on the anode surface during charging, forming lithium dendrites. These dendrites can not only pierce the separator, leading to internal short circuits, but also consume active lithium and reduce battery capacity.
The formation of an unstable SEI film during battery testing can be caused by the following factors:
Poor Electrolyte Quality or Improper Formulation: If the electrolyte contains many impurities, such as moisture, metal ions, or other organic impurities, these substances will participate in reactions during the initial charge-discharge cycles, forming an unstable SEI film. The types and ratios of solvents and lithium salts in the electrolyte significantly impact the SEI film. Improper formulations can lead to instability. (Selecting a high-purity electrolyte can effectively avoid this issue.)
Excessive Charging Voltage: Excessively high charging voltage can cause over-reduction of the electrolyte on the anode surface, potentially forming an SEI film that is too thick or non-uniform, thereby affecting its stability.
Inappropriate Charging Current: Excessively high charging currents can lead to non-uniform intercalation and de-intercalation of lithium ions in the electrode material, producing lithium dendrites or other undesirable morphologies. These issues reduce the cyclable capacity and accelerate battery degradation.
Temperature Effects: High-temperature conditions may decrease the stability of the SEI film, while lower temperatures generally help in forming a dense and stable SEI film. Unsuitable temperature conditions can cause the SEI film structure to reorganize into a porous structure, reducing stability.
Electrode Material Characteristics: The type, structure, and surface properties of the electrode material influence the formation of the SEI film. For example, different carbon materials will form SEI films with different characteristics.
Battery Manufacturing Processes: Improper operations during manufacturing, such as electrode preparation, electrolyte injection, and sealing, can all affect the stability of the SEI film.
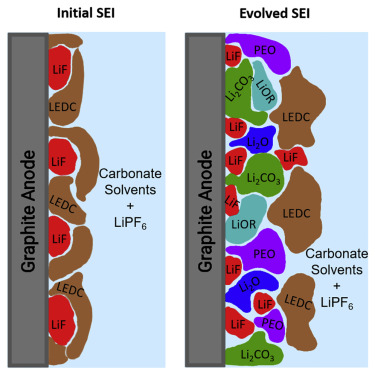
Battery Aging Process: As the number of battery cycles increases, the SEI film may gradually crack and reform, leading to a progressive decline in performance.
External Environmental Factors: Environmental factors such as mechanical pressure and humidity may also lead to SEI film instability.
In general, to obtain a stable SEI film, you can optimize the electrolyte formulation, control charging conditions, select appropriate electrode materials, and improve battery manufacturing processes. Furthermore, future improvements and research on SEI films will focus on developing new electrolytes, designing optimized electrode materials, exploring new SEI formation mechanisms, developing efficient evaluation methods, and promoting the application of artificial intelligence and machine learning. These research directions will provide new breakthroughs and opportunities for the development of lithium-ion batteries, driving further performance enhancements and expanded applications.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
Solid electrolyte interphase (SEI)
Related News:
- Electrochemical Test Techniques for Lithium-ion Batteries: CV, EIS
- Lithium-Ion Battery Core: Electrolyte Composition and Functional Analysis 2024 post
- Design, Assembly, and Testing of Full Coin Cells: Tutorials and Case Studies 2026 post
- 2025 Breaking the fast charging bottleneck! Superwettable Electrolyte Engineering for Fast Charging Li-Ion Batteries
