Why is PVDF unsuitable as a binder for silicon anodes?
I. Silicon Anodes?
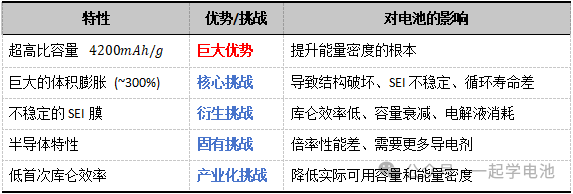

Core Characteristics:
1. Extremely High Theoretical Specific Capacity. Use Neware battery cyclers to test your battery capacity
Based on its alloying reaction mechanism (Li₂₂Si₅), silicon has a theoretical specific capacity as high as approximately 4200 mAh/g. This is more than 10 times that of current commercial graphite anodes (~372 mAh/g, based on intercalation). This is the fundamental reason why research revolves around silicon and is the key to achieving next-generation high-energy-density lithium-ion batteries.
2. Suitable Operating Potential
The lithiation potential of silicon is approximately 0.1–0.5 V (vs. Li/Li⁺), which is higher than the lithium plating potential of graphite (0 V). This makes it less prone to lithium dendrite growth, offering better safety. When paired with high-potential cathode materials, it can provide a higher overall battery operating voltage.
Core Challenges:
1. Massive Volume Effect
Upon full lithiation, the volume expansion of silicon can reach ~300% (for crystalline silicon), the highest among all anode materials. The repeated expansion and contraction generate massive mechanical stress, leading to:
Pulverization of silicon particles.
Loss of electrical contact between particles.
Delamination of active material from the current collector.
2. Unstable Solid Electrolyte Interphase (SEI)
The massive volume changes prevent the SEI film formed on the silicon surface from remaining stable. During each cycle, newly exposed silicon surfaces react with the electrolyte, continuously forming new SEI layers. This leads to:
Infinite thickening of the SEI, which increases interfacial impedance and hinders rate performance.
Clogging of ion channels.
Continuous consumption of active lithium and electrolyte, resulting in low Coulombic efficiency and rapid capacity decay.
3. Poor Intrinsic Conductivity
Silicon is a semiconductor, and its intrinsic electronic and ionic conductivities are far lower than those of graphite. This results in:
Poor rate performance and limited charging/discharging speeds. Test your batteries at a high C rate———-> Neware BTS
Severe polarization, requiring the addition of large amounts of conductive agents, which reduces the overall energy density of the battery.
4. Low Initial Coulombic Efficiency (ICE)
During the first charge (lithiation), a portion of lithium is irreversibly consumed by the formation of the SEI and other side reactions, failing to return to the cathode during discharge. In practical applications, this significantly compromises the reversible capacity and energy density of the battery, representing one of the primary hurdles for industrialization.
II. Why is PVDF Unsuitable as a Binder for Silicon Anodes?
1. Mechanical Property Mismatch
PVDF is a semi-crystalline polymer whose molecular chains are primarily held together by weak Van der Waals forces. Consequently, its mechanical strength and elastic modulus are relatively low. However, silicon anodes undergo massive volume expansion and contraction during the lithiation and delithiation processes, with expansion rates reaching 280%–300%. Therefore, when silicon particles repeatedly expand and contract, the PVDF binder cannot provide sufficient restorative or binding force.
Resulting Issues:
- ① Structural Damage of the Electrode: The binding sites fail, causing the active material (silicon particles) to delaminate from the current collector (copper foil).
② Loss of Electrical Contact: Dislodged silicon particles lose contact with the conductive agent and the current collector, meaning they no longer participate in electrochemical reactions. This leads to a drastic decay in capacity.
③ Formation of “Dead Lithium”: Detached silicon particles become isolated from the entire electrode system, turning into unusable “dead lithium.”
2. Weak Binding Mechanism
The binding mechanism of PVDF primarily relies on physical adsorption and weak Van der Waals forces to adhere to the surface of the active material and the current collector. This force is easily overwhelmed when faced with the massive mechanical stress of silicon, making it impossible to effectively “anchor” the silicon particles to the current collector.
3. Linear Molecular Structure
PVDF molecular chains are linear. They cannot form a three-dimensional, elastic network within the electrode to encapsulate and buffer the volume changes of the silicon particles. Consequently, PVDF is unable to maintain the structural integrity of the electrode effectively.
4. Electrochemical Stability and Conductivity Issues
While PVDF is stable within the voltage range of the cathode, it may undergo minor electrochemical reduction reactions at the low operating potentials of the silicon anode (~0.1–0.5 V vs. $Li/Li^+$), which compromises interfacial stability. Furthermore, PVDF itself is an insulator. Silicon materials already possess poor conductivity, and their volume changes continuously disrupt the conductive network. PVDF cannot assist in conduction—unlike certain conductive polymers (such as PEDOT:PSS)——and may instead act as an obstacle to electron transport.
III. Comparison Between PVDF and the Ideal Binder for Silicon Anodes
The points mentioned above highlight the inherent characteristics of PVDF and the core issues that make it unsuitable as a silicon anode binder. Below, we provide a comparative analysis between PVDF and the ideal binder requirements for silicon anodes:
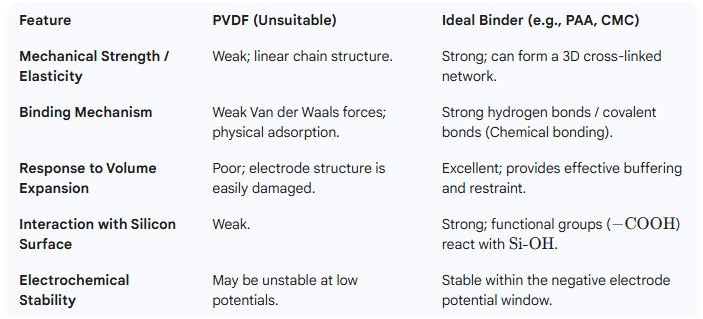
Characteristics of Ideal Binders for Silicon Anodes:
Polyacrylic Acid (PAA): Contains a high density of carboxyl groups ($-COOH$), which can form strong hydrogen bonds and covalent bonds (through esterification) with the silicon surface, providing exceptionally high binding strength.
Sodium Carboxymethyl Cellulose (CMC): A low-cost option that also contains carboxyl groups, effectively enhancing the mechanical stability of the electrode.
Sodium Alginate (Alginate): Extracted from seaweed, its unique “egg-box” cross-linked structure allows it to better accommodate and adapt to large volume changes.
Composite/Self-Healing Binders: These combine different polymers or functional materials to simultaneously satisfy multiple requirements, such as high adhesion, superior elasticity, and self-healing capabilities.
Summary of Reasons Why PVDF is Unsuitable as a Silicon Anode Binder
Insufficient Mechanical Strength (Low Modulus): PVDF consists of linear molecular chains that cannot withstand the massive stress generated by silicon’s >300% volume change. This inevitably leads to the pulverization of the electrode structure.
Weak Binding Mode: It relies primarily on physical adsorption and Van der Waals forces, which are significantly weaker than the hydrogen bonding and covalent bonding provided by PAA/CMC. Consequently, active material particles easily delaminate and fall off.
Lack of Elasticity: PVDF cannot pull the active material back to its original position during volume contraction, failing to maintain a stable and continuous conductive network.
Electrochemical Stability Issues: At the low operating potentials of the silicon anode, PVDF may undergo minor reductive decomposition, which further destabilizes the SEI (Solid Electrolyte Interphase) film.
Conclusion
The “hard glue” style of PVDF—characterized by its linear structure and weak bonding—is completely incompatible with an active material like silicon that undergoes such “violent breathing” (expansion/contraction). This is why, in silicon anode research, PVDF is almost never considered. Instead, researchers must seek out “spider-web” style binders that possess both high adhesion strength and superior elasticity.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
