Zn//MnO2 battery from Primary to Rechargeable: A Timeline of Key Breakthroughs in Aqueous Zinc-Ion Batteries (AZIBs)
For renewable energy to be truly viable, safe, low-cost, and scalable energy storage is essential. Aqueous Zinc-Ion Batteries (AZIBs) have entered the spotlight due to their non-flammable nature, eco-friendly raw materials, and high ionic conductivity. Through a comprehensive timeline, this article briefly outlines the critical milestones and methodologies that transformed the Zn//MnO2 (Zinc-Manganese) system from a primary cell into a rechargeable powerhouse.
Why is everyone refocusing on our “old friend,” the zinc-manganese battery? The answer goes beyond mere “safety and affordability.” When researchers shifted their focus to the crystal framework of MnO2 and the chemical windows of aqueous electrolytes, a series of new phenomena were illuminated: higher voltage plateaus, controllable cycle life, and clearer energy storage mechanisms. We invite you to follow this timeline—from the primary cells of 1866 to the modern rechargeable era—to see how each breakthrough has reshaped the landscape of AZIBs today.
Zn//MnO2 battery—>Aqueous Zinc-Ion Batteries (AZIBs)
01 The Origin (Voltaic Pile) → 1866 | Primary Alkaline Zn–Mn
1866: The Leclanché Cell: Composed of a zinc metal anode, an $MnO_2$ cathode, and an aqueous electrolyte, the Leclanché cell featured a simple structure and low cost. However, its irreversible reactions and difficult-to-control dry-pressed electrode structures formed the industrial prototype of the primary alkaline dry cell.
The typical electrochemical reaction of a primary alkaline Zn–Mn battery involves the oxidation of zinc, which releases electrons, while the manganese dioxide acts as the cathode, accepting these electrons and undergoing a reduction reaction to produce lower manganese oxidation states. Due to its relatively simple design and reliable performance, the primary alkaline Zn–Mn battery became ubiquitous in consumer electronics.
Nevertheless, the manufacturing process prioritized low cost as its defining attribute, primarily utilizing dry-pressing for electrode preparation. While intuitive, this process often resulted in poor control over electrode porosity and homogeneity. Furthermore, the chemical reactions within these cells are inherently irreversible; once discharged, they cannot be reused.
02 1986 | The Rechargeable Prototype in Neutral ZnSO₄
In 1986, Japanese scientist Takakazu and his team developed a Zn//MnO2 battery using a neutral ZnSO4 electrolyte. This system achieved approximately 30 reversible cycles in a neutral environment, successfully providing a “proof of concept” that aqueous rechargeable zinc batteries were functionally viable.
However, because the underlying electrochemical mechanisms remained unclear at the time, the research received limited attention from the broader scientific community.
03 2009 | First Direct Observation of Reversible Zn2+ Intercalation/De-intercalation in MnO2
Ref: Chengjun Xu et al., 2009, Electrochem. Solid-State Lett. 12, A61
Key Achievement: Professor Feiyu Kang’s research group at Tsinghua University provided the first concrete mechanistic evidence: the reversibility of ion intercalation was experimentally confirmed.
2012 | Reintroduction of the AZIB Concept & The & α-MnO2 Milestone. Zn//MnO2 battery—>Aqueous Zinc-Ion Batteries (AZIBs)
Ref. Xu, C., Li, B., Du, H. and Kang, F. (2012), Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery†. Angew. Chem. Int. Ed., 51: 933-935. https://doi.org/10.1002/anie.201106307
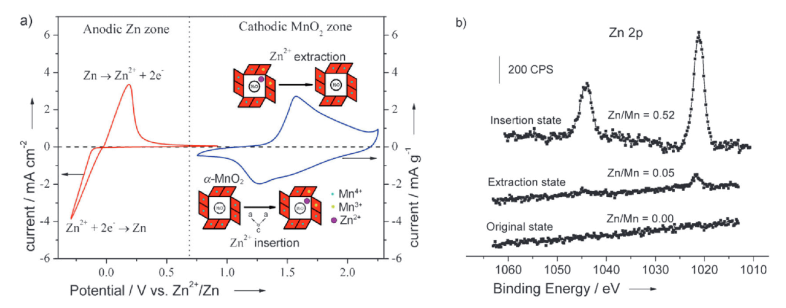
Figure 1. a) Cyclic voltammograms (CV) of a Zn anode (red line) and an α-MnO2 cathode (blue line) at 2 mVs-1 in a 0.1 mol L-1 Zn (NO3) 2 aqueous electrolyte (pH 5.2). The anodic and cathodic processes of the zinc-ion battery are shown respectively. b) Zn 2p core-level spectra of the crystalline α-MnO2 cathode in its pristine, extracted, and inserted states.
The term “Aqueous Zinc-Ion Battery” was officially redefined, following the discovery of the Zn2 + intercalation reaction in MnO2 using ZnSO4 or Zn (NO3) 2 electrolytes—marking the first formal introduction of the AZIB concept. Since then, research and applications of manganese-based oxides (such as MnO2/Mn2O3/Mn3O4, etc.) in AZIBs have experienced explosive growth. Among these, MnO2 is regarded as a premier candidate for next-generation zinc storage materials due to its high theoretical capacity (308 mAh g-1 based on single-electron Mn4+/Mn3+ transfer) and high operating voltage (approximately 1.3 V vs Zn2+/Zn). The Zn/α-MnO2 system served as the starting point for high-energy-density development, driving a “dual-track” research strategy focused on “Cathode Optimization + Electrolyte Engineering.”
04 2015 | γ-MnO2: The Launchpad for Exploring Electrochemical Reaction Principles Across Different MnO2 Polymorphs
Ref. Muhammad H. Alfaruqi, Vinod Mathew, Jihyeon Gim, Sungjin Kim, Jinju Song, Joseph P. Baboo, Sun H. Choi, and Jaekook Kim Chemistry of Materials 2015 27 (10), 3609-3620 DOI: 10.1021/cm504717p
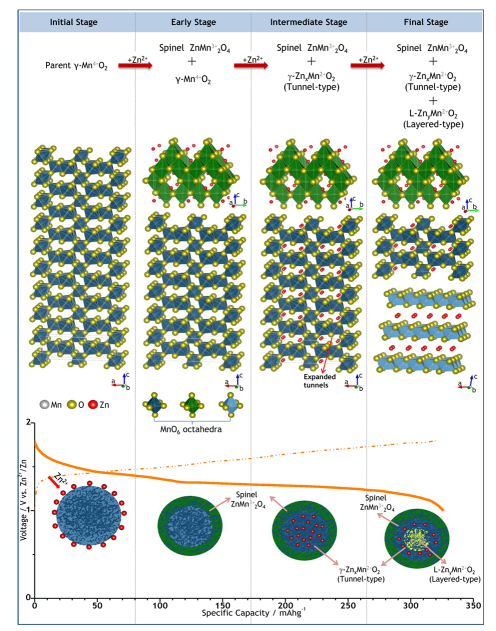
This study provides an in-depth investigation into the structural transformation of mesoporous γ-MnO2 cathodes in Zinc-Ion Batteries (ZIBs) during electrochemical reactions. Combined in-situ synchrotron XANES and XRD studies reveal that the tunnel-type parent γ-MnO2 undergoes a structural evolution into a spinel-type Mn(III) phase (ZnMn2O4) and two new intermediate Mn(II) phases—namely, tunnel-type γ-ZnxMnO2 and layered L-ZnyMnO2. These phases, characterized by multiple oxidation states, coexist upon completion of electrochemical Zn insertion. During the subsequent Zn de-insertion/extraction process, most of these multi-oxidation-state phases were observed to revert to the parent γ-MnO2 phase.
Depending on the different connection modes of MnO6 octahedra, manganese dioxide exists in various crystalline forms (e.g., ε-,α-,β-,γ-,λ-,γ-,σ- and δ- types). Furthermore, due to variations in preparation conditions, MnO2 exhibits diverse morphologies, including nanoparticles, nanodots, nanospheres, nanorods, nanowires, and nanosheets. These distinct crystal polymorphs and morphologies endow them with unique electrochemical performance in AZIBs.
05 2019 | High-Voltage Electrolytic Zn//MnO2 System. Zn//MnO2 battery—>Aqueous Zinc-Ion Batteries (AZIBs)
Ref. D. Chao, W. Zhou, C. Ye, Q. Zhang, Y. Chen, L. Gu, K. Davey, S.-Z. Qiao, Angew. Chem. Int. Ed. 2019, 58, 7823.
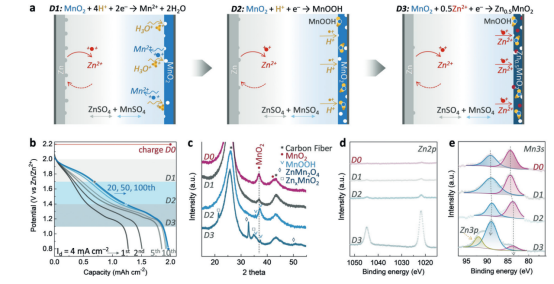
Figure 3. Schematic and charge storage mechanism of the MnO2-Zn ion battery in 1 m ZnSO4 and 1 m MnSO4 electrolytes. a) Galvanostatic discharge process, including steps D1, D2, and D3. b) Galvanostatic discharge curves during the first 100 cycles. The three shaded areas represent the three charge-transfer steps corresponding to D1, D2, and D3. c–e) Ex-situ XRD and XPS patterns of the MnO2 cathode at different depths of discharge (DOD) during initial discharge: D0 (fully charged), D1 (1.7 V), D2 (1.4 V), and D3 (0.8 V). d, e) High-resolution XPS of Zn 2p (d) and Mn 3s (e). Open circles correspond to experimental spectra; blue and red curves represent the fitting results of the Mn 3s spectra. The yellow curve is the fitting component of the Zn 3p peak.
This work proposes a potential high-voltage MnO2 electrolytic process within conventional zinc-ion batteries and reports a novel electrolytic Zn-MnO2 system. Figure 3 illustrates the mechanism of in-situ electrodeposition coupled with partitioned discharge:
Initially, at a constant potential of 2.2 V, Mn2+ is oxidatively deposited as epsilon-MnO2 onto carbon fibers (using Zn foam as the anode), achieving high deposition efficiency (approx. 2 mAh/cm2 in 90 s). During galvanostatic cycling, three distinct discharge stages appear: D1 (2.0-1.7 V), D2 (1.7-1.4 V), and D3 (1.4-0.8 V).
For the D1 stage, in-situ and ex-situ characterizations (XRD/XPS) remain largely unchanged, with Mn remaining in the +4 state. This suggests that D1 is primarily dominated by electrolytic or interface processes rather than bulk valence changes. Moving into the D2 stage, an enhancement of the Mn-OH signal in the O 1s spectrum is observed, accompanied by H+ intercalation to form MnOOH. By the D3 stage, signals for ZnxMnO2 and Zn2MnO4 emerge, and the Mn 3s splitting energy increases to approximately 5.09 or 5.28 eV, indicating Zn2+ intercalation and a subsequent reduction in the Mn valence state.
Overall mechanism: The system starts with the in-situ growth and activation of the cathode, followed by the interface-dominated high-voltage D1 stage, and finally, the sequential contribution of H+ and Zn2+ intercalation to the capacity in the lower voltage regions.
By enabling proton and electron kinetics, the electrolytic process has been maximized. Compared to other zinc-based electrochemical devices, this new electrolytic Zn-MnO2 battery delivers a record-high output voltage of 1.95 V, an impressive gravimetric capacity of approximately 570 mAh/g, and a record energy density of about 409 Wh/kg based on the mass of both anode and cathode active materials. At a conservative estimate, the cost is less than 10 dollars per kWh.
06 2021 | Synergetic Intercalation of Non-Metallic Ions
Ref. S. Wang, Z. Yuan, X. Zhang, S. Bi, Z. Zhou, J. Tian, Q. Zhang, Z. Niu, Angew. Chem. Int. Ed. 2021, 60, 7056.
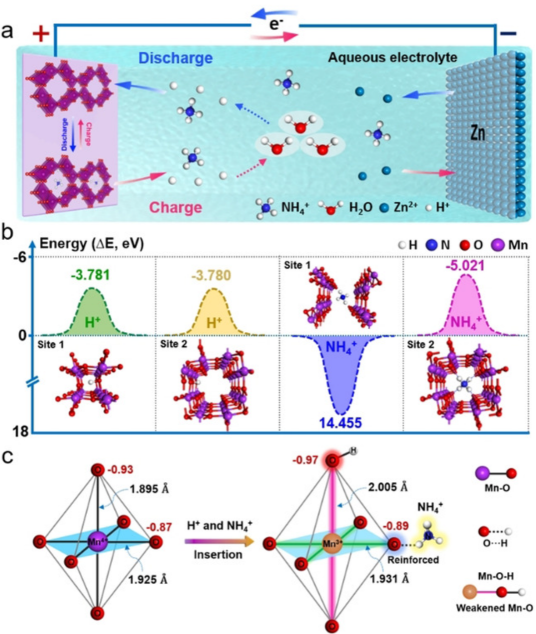
Figure 4. a) Schematic illustration of the H+/NH4+ ion co-insertion chemistry mechanism in aqueous Zn/MnO2 battery systems. b) Insertion energies of H+ or NH4+ ions in MnO2 calculated via first-principles methods. c) Local geometry and charge distribution of MnO2 after the insertion of H+ and NH4+ ions.
Dual-ion co-insertion typically enhances the electrochemical performance of aqueous zinc-ion batteries. Although the co-insertion of non-metallic ions has been achieved in these systems, mastering the chemistry of non-metallic cation co-insertion remains a challenge. Niu Zhiqiang’s research group at Nankai University has developed a reversible H+/NH4+ co-insertion and extraction mechanism within the aqueous Zn/MnO2 battery system.
The synergistic effect between these two ions endows the aqueous zinc battery with rapid ion diffusion kinetics and reversible structural evolution of the MnO2 cathode. Consequently, the Zn/MnO2 battery demonstrates superior rate capability and cycling stability.
From primary cells to rechargeable systems, every leap in zinc-manganese battery technology has originated from the collaborative design of structure, electrolyte, and interface. For researchers, the strategic choices regarding MnO2 polymorph selection, the establishment of dual electron/ion pathways, and the mitigation of side reactions within the electrolyte determine the final triangular balance between energy density, rate performance, and cycle life.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
If you neet to test your battery’s performance and cycle life, please contact Neware.
Neware battery cyclers/battery testers
More News: Neware NEWs
Related News:
- How do aqueous zinc-ion batteries relate to traditional Zn//MnO₂ electrochemical systems? 2026 post
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-2
- Electrochemical Test Techniques for Lithium-ion Batteries: CV, EIS
- A Bifunctional Separator with Gradient Distribution of MCM-41 Zeolite for High-Performance Aqueous Zinc-ion Batteries