Synergistic Coupling of Host and Electrolyte Achieving1270 Wh/L in Anode-Free Lithium Metal Batteries. Anode-Free Battery Doubles Electric Vehicle Driving Range.
[POSTECH, KAIST, and Gyeongsang National University achieve a record-breaking energy density of 1,270 Wh/L]
Could an electric vehicle travel from Seoul to Busan and back on a single charge? Could drivers stop worrying about battery performance even in winter? A Korean research team has taken a major step toward answering these questions by developing an anode-free lithium metal battery that can deliver nearly double driving range using the same battery volume.
A joint research team led by Professor Soojin Park and Dr. Dong-Yeob Han of the Department of Chemistry at POSTECH, together with Professor Nam-Soon Choi and Dr. Saehun Kim of KAIST, and Professor Tae Kyung Lee and researcher Junsu Son of Gyeongsang National University, has successfully achieved a volumetric energy density of 1,270 Wh/L in an anode-free lithium metal battery. This value is nearly twice that of current lithium-ion batteries used in electric vehicles, which typically deliver around 650 Wh/L. The achievement was published as a Front Cover article in Advanced Materials.
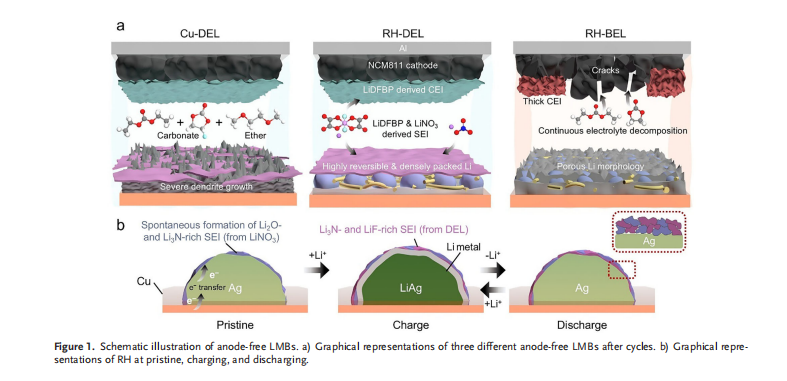
An anode-free lithium metal battery eliminates the conventional anode altogether. Instead, lithium ions stored in the cathode move during charging and deposit directly onto a copper current collector. By removing unnecessary components, more internal space can be devoted to energy storage, much like fitting more fuel into the same-sized tank. However, this design comes with serious challenges. If lithium deposits unevenly, sharp needle-like structures known as dendrites can form, increasing the risk of short circuits and potential safety hazards. Repeated charging and discharging can also damage the lithium surface, rapidly shortening battery life.
To address these issues, the research team adopted a dual strategy combining a Reversible Host (RH) and a Designed Electrolyte (DEL). The reversible host consists of a polymer framework embedded with uniformly distributed silver (Ag) nanoparticles, guiding lithium to deposit in designated locations rather than randomly. In simple terms, it acts like a dedicated parking lot for lithium, ensuring ordered and uniform deposition.
The designed electrolyte further enhances stability by forming a thin but robust protective layer composed of Li₂O and Li₃N on the lithium surface. This layer functions like a bandage on skin, preventing harmful dendrite growth while maintaining open pathways for lithium ions transport.
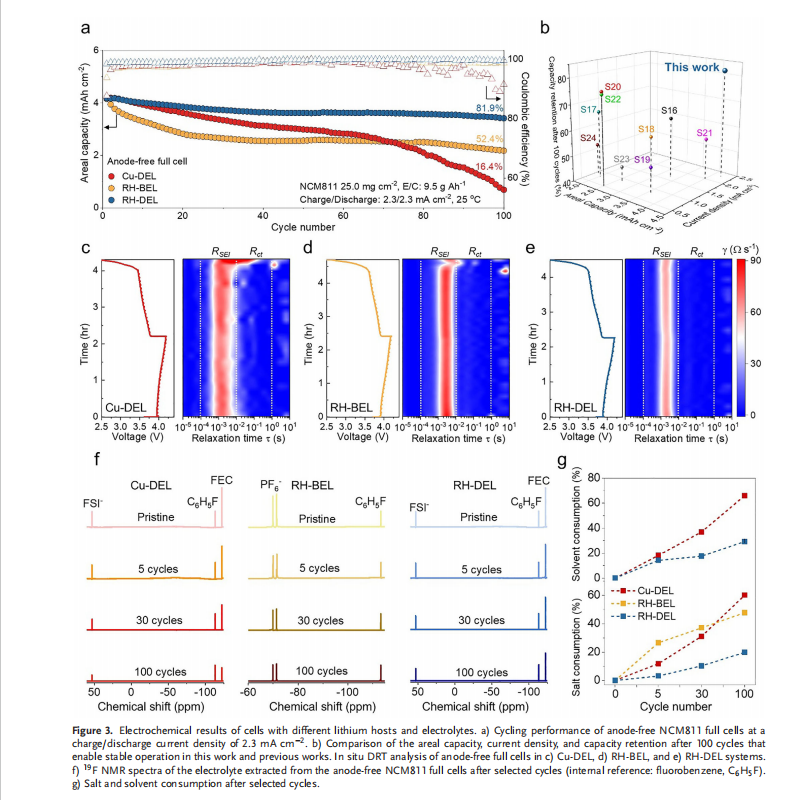
When combined, the RH–DEL system delivered outstanding performance. Under high areal capacity (4.6 mAh cm⁻²) and current density (2.3 mA cm⁻²), the battery retained 81.9% of its initial capacity after 100 cycles and achieved an average Coulombic efficiency of 99.6%. These results enabled the team to reach the record-breaking 1,270 Wh/L volumetric energy density in anode-free lithium metal batteries.
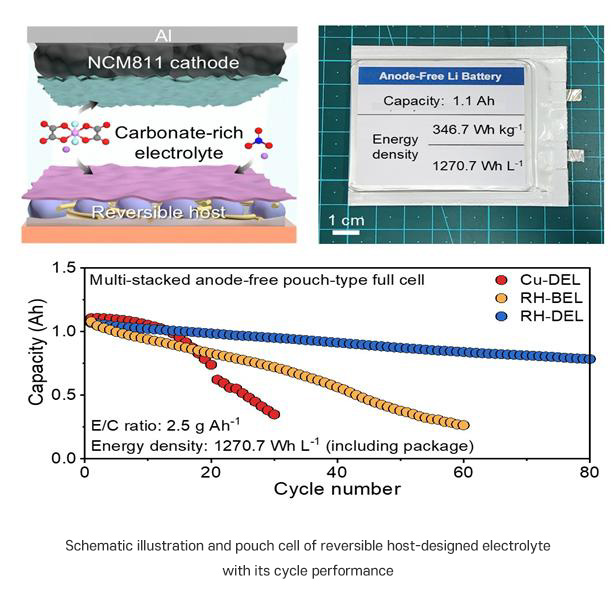
Importantly, this performance was validated not only in small laboratory cells but also in pouch-type batteries, which are closer to real-world electric vehicle applications. Even with a minimal amount of electrolyte (E/C = 2.5 g Ah⁻¹) and under low stack pressure (20 kPa), the batteries operated stably. This demonstrates strong potential for reducing battery weight and volume while lowering manufacturing burdens, significantly improving commercial viability.
Professor Soojin Park commented, “This work represents a meaningful breakthrough by simultaneously addressing efficiency and lifetime issues in anode-free lithium metal batteries.” Professor Tae Kyung Lee added, “Our study demonstrates that electrolyte design based on commercially available solvents can achieve both high lithium-ion mobility and interfacial stability.”
This research was supported by the Ministry of Science and ICT (MSIT) of Korea.
More details: Anode-free lithium metal batteries (LMBs) represent a promising avenue for maximizing energy density by eliminating excess lithium (Li), yet their practical implementation is impeded by limited Li reversibility and pronounced interfacial instability. Herein, a synergistic coupling strategy is reported that integrates a highly reversible host (RH) with a rationally designed, carbonate-rich electrolyte (DEL) to concurrently address these fundamental challenges. The RH spontaneously induces the formation of a robust Li 2 O- and Li 3 N-rich solid electrolyte interphase via electron transfer, which effectively accommodates Li volume changes and suppresses dendritic growth. Complementarily, DEL, composed of commercially available salts, solvents, and additives, establishes stable electrode-electrolyte interphases at both electrodes. Coin-type anode-free full cells employing the RH-DEL configuration achieve an average Coulombic efficiency of 99.6% and 81.9% capacity retention after 100 cycles at 4.6 mAh cm−2 and 2.3 mA cm−2 . Stacked pouch-type full cells further deliver a record volumetric energy density of 1270 Wh L−1 (including packaging) under lean electrolyte (E/C = 2.5 g Ah−1 ) and a low stack pressure(≈20 kPa). This synergistic approach delineates a practical pathway toward high-energy, long-life anode-free LMBs for advanced energy storage systems.
Entire article DOI: https://doi.org/10.1002/adma.202515906
More posts: NEWARE NEWS
Related News:
- Lithium Ion vs Lithium Polymer: A Comprehensive Comparison Guide for 2024
- Power Battery Tech: Latest Advances & Future Trends
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-1
- Design, Assembly, and Testing of Full Coin Cells: Tutorials and Case Studies 2026 post