Breaking the fast charging bottleneck! Shanghai Jiao Tong University & Southern University of Science and Technology join forces: Super-wetting electrolyte reshapes the SEI layer, comprehensively improving lithium battery performance.
Recently, Professor Wan Jiayu’s team at Shanghai Jiao Tong University, in collaboration with Professors Liu Ke, Luo Guangfu, and Deng Yonghong from Southern University of Science and Technology, published a groundbreaking study in the top journal ACS Energy Letters (IF: 22.0). They proposed a general “stepwise liquid injection” interface engineering strategy, successfully constructing a uniform, stable, and inorganic-rich SEI layer on a graphite anode using an ultra-low concentration of superwetting electrolyte. This breakthrough overcomes multiple bottlenecks in wettability, SEI stability, and fast-charging performance of traditional electrolytes.

01 The Dilemma: The SEI Layer, a Double-Edged Sword for Lithium-ion Battery Performance
To understand the value of this research, we must first understand the SEI layer.
During the initial charging (formation) of a lithium-ion battery, certain components in the electrolyte undergo irreversible reduction and decomposition on the surface of the graphite anode, forming a passivation film covering the electrode surface—this is the SEI layer.
An ideal SEI layer plays multiple crucial roles: it acts like a robust gate, allowing lithium ions to smoothly insert and extract while preventing the continuous decomposition of the electrolyte; it also acts like an elastic armor, adapting to the volume changes of the electrode material during charging and discharging, maintaining the electrochemical stability of the interface.
Graphite, due to its low cost and suitable operating potential (~0.1 V vs. Li/Li⁺), is currently the dominant anode material in commercially available lithium-ion batteries. However, it is precisely on the graphite surface that the inherent defects of the SEI layer are amplified.
Suboptimal SEI layers primarily stem from two issues:
First, incomplete coverage. Due to poor wettability at the electrolyte-graphite electrode interface, the formed SEI film may not completely and uniformly cover the surface of all graphite particles, leaving localized “holes.”
Second, unstable composition. Traditional electrolyte-formed SEI layers have a high proportion of organic components. These components have poor mechanical strength, low ionic conductivity, and are prone to dissolution and reconstruction during cycling.
These two defects collectively lead to a series of chain reactions: uneven lithium-ion transport, repeated breakage and repair of the SEI layer during cycling, irreversible consumption of active lithium, and a continuous increase in interfacial resistance.
The ultimate result is: reduced battery cycle life, a high risk of lithium plating during fast charging (forming dendrites that puncture the separator and cause short circuits), and potential safety hazards such as thermal runaway and even fire and explosion.
Traditional solutions often focus on electrolyte engineering, such as adding film-forming additives and using high-concentration electrolytes. However, these methods often bring new problems: significantly increased viscosity leading to wetting difficulties, high costs, and deterioration in low-temperature performance. It is difficult to achieve a balance between these factors.
02 Breaking the deadlock: A “super-wet” key unlocks two locks
A joint team from Shanghai Jiao Tong University and Southern University of Science and Technology (SUSTech) has taken a novel approach—manipulating the physicochemical properties of electrolytes, particularly their wetting behavior and electrochemical window.
The core of their innovation lies in a stepwise electrolyte injection strategy for battery interfaces: during the initial electrolyte injection, a super-wetting electrolyte with an extremely low concentration (0 M or 0.02 M LiPF₆) is used specifically for the battery formation stage to construct the initial SEI layer; subsequently, a conventional concentration of electrolyte is added for normal charge-discharge cycling.
This “super-wetting” key cleverly unlocks two long-standing challenges in the industry.
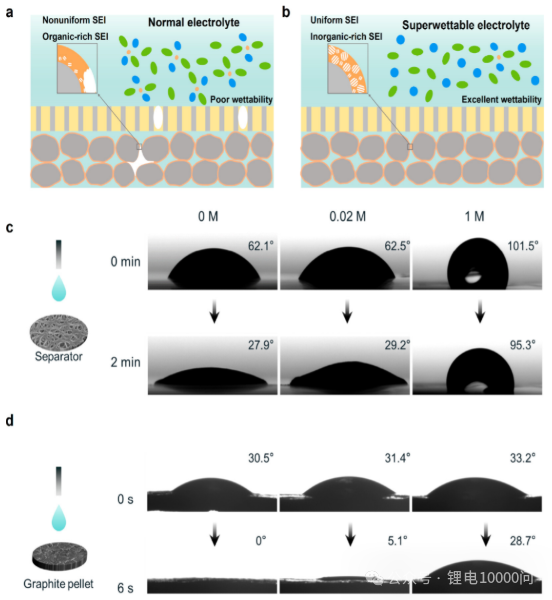
The first challenge: interfacial wettability. The research team visually demonstrated the “superpower” of the ultra-low concentration electrolyte through contact angle testing. As shown in Figure 1(c-d), the 0 M and 0.02 M electrolytes spread almost instantaneously on the polyethylene (PE) separator and graphite sheet surfaces, exhibiting extremely small contact angles and perfect superhydrophilicity.
In contrast, conventional 1 M electrolytes exhibit significant hydrophobicity, with droplets “curling up” into spherical shapes on the surface. This excellent wettability ensures that the electrolyte can quickly and uniformly wet every pore and surface of the electrode and diaphragm, laying the physical foundation for the formation of a uniform and complete SEI coating.
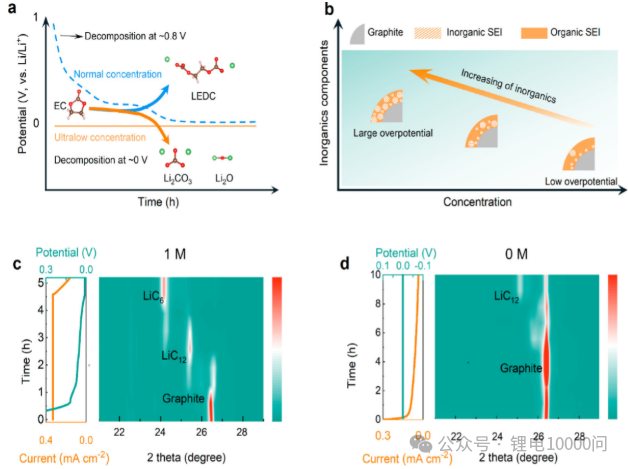
The second key factor: SEI chemical composition. An ideal SEI should be rich in inorganic compounds such as LiF, Li₂O, and Li₂CO₃. These components possess higher mechanical strength, better ionic conductivity, and superior chemical/electrochemical stability. The traditional challenge lies in the fact that driving the electrolyte decomposition to generate these inorganic compounds typically requires a high reduction potential (i.e., a high overpotential).
However, in conventional high-concentration electrolytes, applying a high overpotential often means an excessively large formation current, leading to an overly vigorous and uneven reduction reaction, resulting in a thick, uneven, and organic-rich inferior SEI.
The research team’s ingenuity lies in utilizing the inherently low conductivity of ultra-low concentration electrolytes. Under the same formation regime, using ultra-low concentration electrolytes spontaneously generates a significantly higher overpotential (as shown in Figure 2(b)).
This “high overpotential, low current” formation mode is perfect: the high overpotential is thermodynamically favorable for the generation of inorganic components; while the extremely low current density ensures a smooth and uniform reaction, thereby constructing a thin, dense, and high-quality SEI layer rich in inorganic matter on the graphite surface.
03 Validation: Comprehensive Performance Improvement, From Half-Cell to Full-Cell
Theoretical concepts require experimental data to support them. The research team comprehensively validated the superiority of this strategy through systematic electrochemical testing and physical characterization.
Leap in Half-Cell Performance:
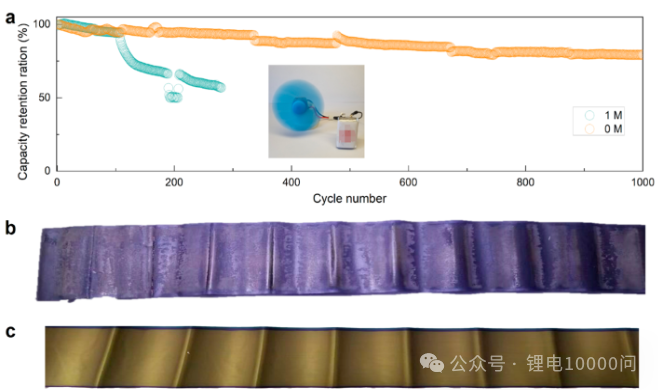
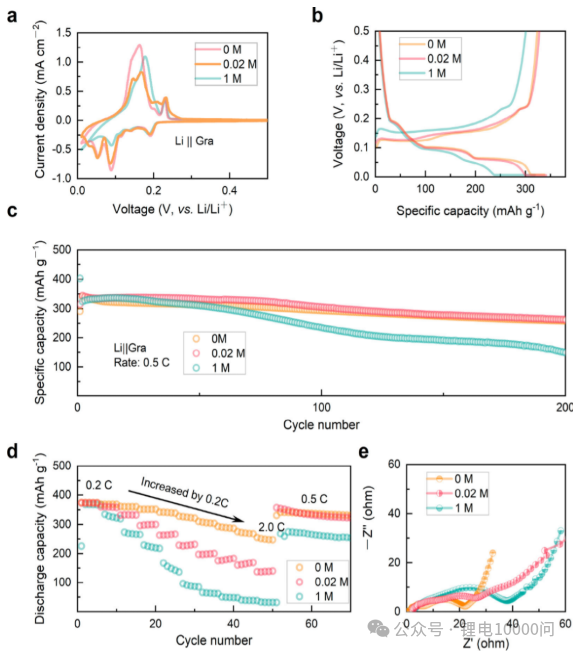
- Cycle Stability: Li||graphite half-cells formed using 0 M and 0.02 M electrolytes exhibit significantly higher capacity retention after 200 cycles at 0.5 C than the battery with 1 M electrolyte (the latter retains only 44.7% capacity after 100 cycles).
- Rate Performance: At a high rate of 5 C, the ultra-low concentration electrolyte battery still delivers a high specific capacity of 166 mAh g⁻¹, demonstrating excellent fast charge-discharge capability.
- Interfacial Kinetics: Electrochemical impedance spectroscopy (EIS) shows that the battery formed with the ultra-low concentration electrolyte exhibits significantly lower charge transfer resistance (Rct) and SEI film resistance (R_SEI), indicating more rapid lithium-ion transport at the interface.
Impressive Full-Battery Performance:
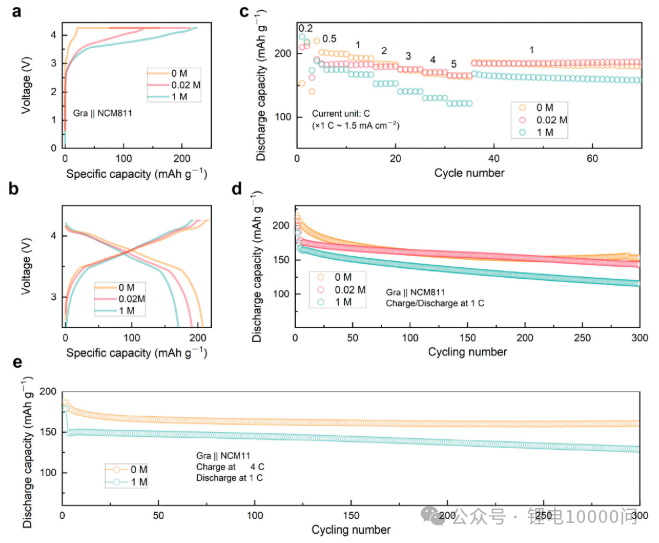
The study further validated the practicality of this strategy in graphite||NCM811 (nickel-cobalt-manganese ternary material) pouch full-cell batteries.
- Long Cycle Life: Full cells fabricated using 0 M and 0.02 M electrolytes achieved capacity retention of 76.6% and 75.6% respectively after 300 cycles at 1 C, significantly outperforming the 66.1% of the 1 M electrolyte group.
- Superior Fast Charging Capability: Cycling at an extremely high charge rate of 4 C (i.e., fully charged in 15 minutes), the ultra-low concentration electrolyte group maintained a capacity retention of up to 89% after 300 cycles, while the control group’s capacity had already decayed to a low level. This directly demonstrates the technology’s strong adaptability to fast-charging scenarios.
Test Half-Cell and Full-Battery Performance, use Neware battery cyclers.
04 Insight: Characterization Reveals the Root of Success—Uniform and Inorganic-Rich SEI
The performance improvement stems from a fundamental change in the interface. The research team used a series of advanced characterization techniques to uncover the microscopic secrets behind the success.
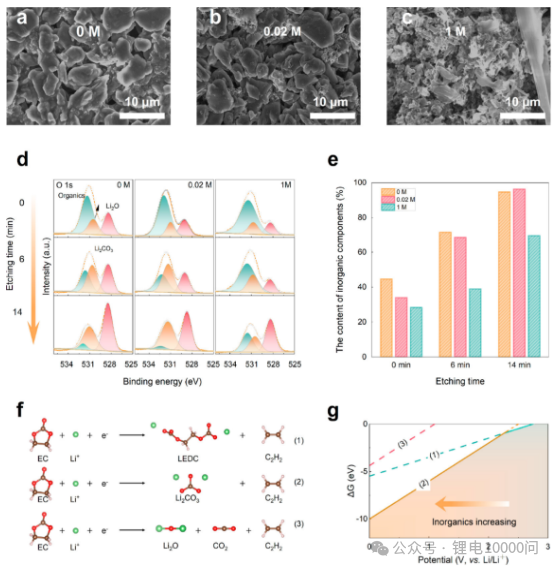
Morphological Observation (SEM): After 200 cycles, the graphite anodes using 0 M and 0.02 M electrolytes exhibited uniform and smooth surfaces, displaying a uniform yellow color indicating complete lithiation (Fig. 4a-b). In contrast, the anode using 1 M electrolyte showed uneven coloring, with a blue hue representing lithium deposition in the central region. SEM revealed obvious lithium dendrites and breakage (Fig. 4c), providing direct evidence of fast-charging failure and safety hazards.
Compositional Analysis (XPS): In-depth X-ray photoelectron spectroscopy provided decisive evidence. Fitting analysis of the O 1s spectrum showed that the signal intensity of inorganic components (such as Li₂O) was significantly higher in the SEI layer formed by ultra-low concentration electrolytes (Figures 4d-e). Calculations of the atomic ratio of Li₂O to C-O-containing organic species confirmed that low-concentration electrolytes promoted the formation of an inorganic-rich SEI.
Mechanism Calculations (DFT): To provide a theoretical explanation, the team calculated the Gibbs free energy changes of the decomposition pathway of EC (ethylene carbonate), a common solvent in the electrolyte, at different potentials (Figures 4f-g). The calculations showed that at higher reduction potentials (overpotentials), the decomposition of EC to produce inorganic compounds such as Li₂CO₃ and Li₂O is thermodynamically dominant, theoretically supporting the conclusion that high overpotentials promote the formation of an inorganic-rich SEI.
05 Outlook: A Potential Path from Laboratory Innovation to Industrial Transformation
This research not only provides a concrete technical solution but also pioneers a new paradigm for precisely designing electrode interfaces by controlling the physicochemical properties of the electrolyte.
The strategy of “stepwise electrolyte injection, wet-then-concentrate” isolates the core step of SEI construction for “precision surgery,” achieving synergistic optimization of SEI morphology and composition.
Its potential advantages and far-reaching impact include:
- High versatility: Theoretically, this strategy is applicable to various anode materials (such as silicon-based and lithium metal anodes) and electrolyte systems, providing a new approach to solving interface challenges in other battery systems.
- Good process compatibility: Stepwise electrolyte injection is easily integrated into existing battery manufacturing processes without requiring disruptive modifications to existing production lines, resulting in relatively low barriers to industrialization.
- Controllable cost: Although a special ultra-low concentration electrolyte is used, its usage is limited to the formation stage, resulting in a limited increase in overall cost while providing significant performance improvements.
- Directly addressing industry pain points: The research findings directly respond to the urgent needs of electric vehicles for fast charging and long-life batteries. The verification of maintaining long-cycle stability under 4C fast charging provides a strong technical candidate for developing next-generation power batteries that offer “400km range with 10-15 minutes of charging.”
From: Superwettable Electrolyte Engineering for Fast Charging Li-Ion Batteries
Related News:
- 3 mins to know fast charging battery technology
- Lithium Ion vs Lithium Polymer: A Comprehensive Comparison Guide for 2024
- Lithium-Ion Battery Core: Electrolyte Composition and Functional Analysis 2024 post
- How do the batteries in Xiaomi phones, Apple phones, and Tesla electric vehicles achieve fast charging?