All Solid State Battery with Soft Carbon–TiSi2 Multilayer Structure for Optimized LiSi Anodes

Sulfide-based all-solid-state batteries, with their excellent safety and high theoretical energy density, are considered a key development direction for next-generation high-energy-density energy storage technology. However, their practical application is limited by the performance shortcomings of anode materials: traditional graphite anodes are difficult to match the ever-increasing energy density requirements, while lithium metal anodes are plagued by dendrite growth and interfacial side reactions. Against this backdrop, silicon-based anodes, with their significant advantages of ultra-high theoretical capacity (3579 mAh g⁻¹) and low operating potential (0.4 V vs. Li⁺/Li), have become a highly promising alternative. In recent years, research on lithium-silicon alloy anodes in sulfide-based all-solid-state battery systems has achieved many breakthroughs—for example, by introducing hard carbon to build a three-dimensional conductive network, or by using pre-lithiation strategies to significantly improve the first-cycle coulombic efficiency and cycle stability. These achievements have all confirmed the feasibility of this technical route. Despite this, the application of lithium-silicon anodes still faces three major challenges: first, the problem of volume expansion, where silicon and lithium-silicon alloys undergo drastic volume expansion and contraction during cycling, leading to contact failure at the electrode-electrolyte interface; second, the issue of chemical compatibility, where silicon undergoes severe interfacial side reactions with sulfide solid electrolytes during cycling, resulting in increased interfacial impedance; and third, the risk of lithium dendrite growth, where abundant lithium sources in lithium-silicon anodes may induce lithium dendrites to penetrate the electrolyte layer, causing a short circuit in the battery. These core bottlenecks severely hinder the development of high-performance sulfide all-solid-state batteries. Therefore, developing innovative anode structures that can simultaneously address the problems of volume expansion, interfacial side reactions, and lithium dendrite formation has become an urgent need and a core research focus in the field.
Core Content Interpretation
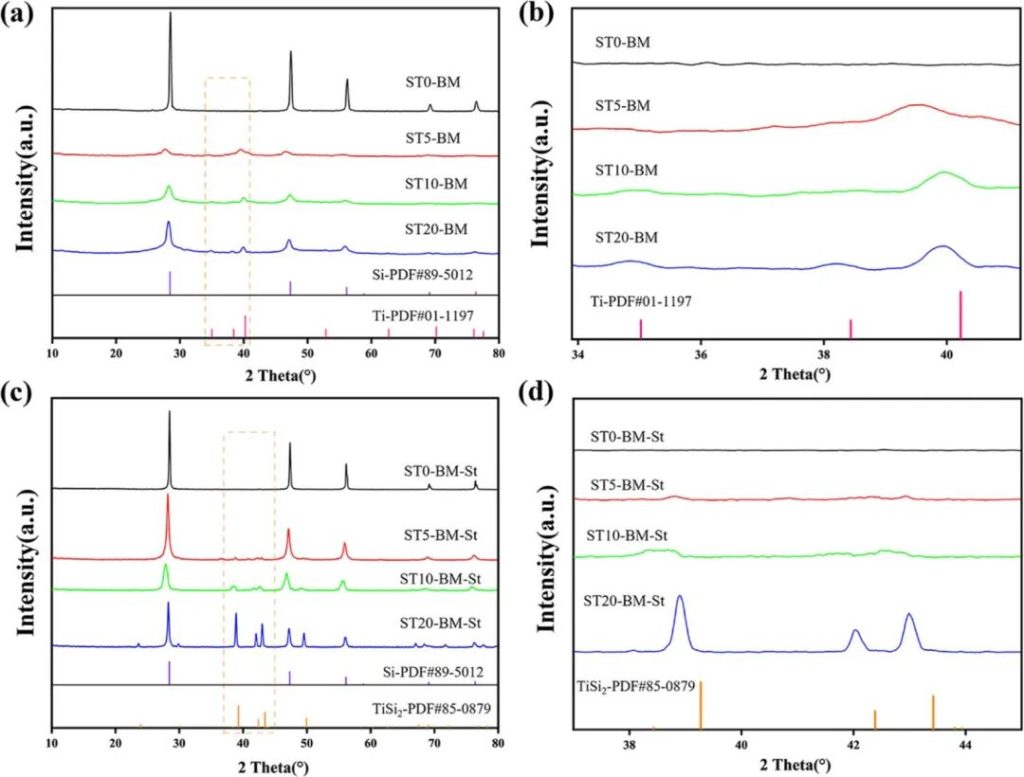
Figure 1 | a) XRD pattern of ST0/5/10/20-BM; b) Enlarged view of the selected area in (a); c) XRD pattern of ST0/5/10/20-BM-St; d) Enlarged view of the selected area in (c).
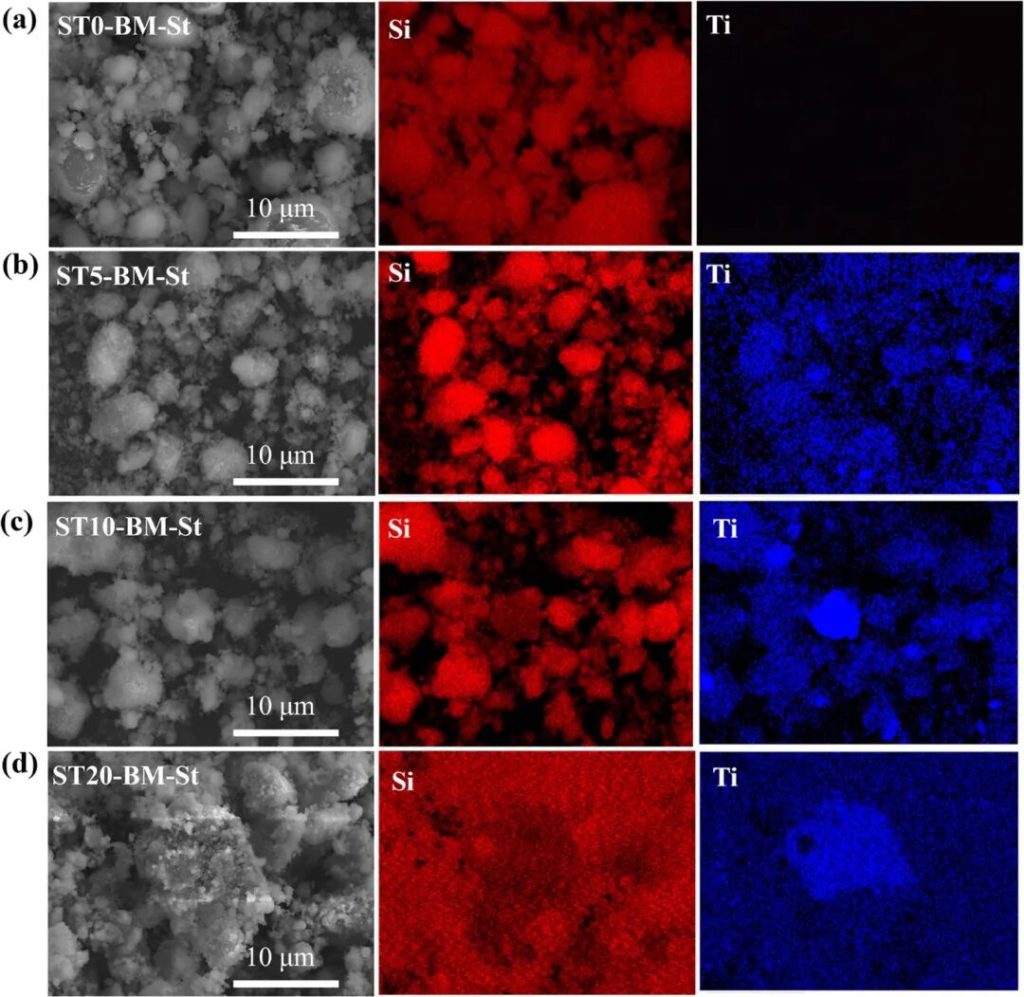
Figure 2 | SEM images and corresponding EDS element distribution maps of ST0-BM-St, b) ST5-BM-St, c) ST10-BM-St, d) ST20-BM-St.
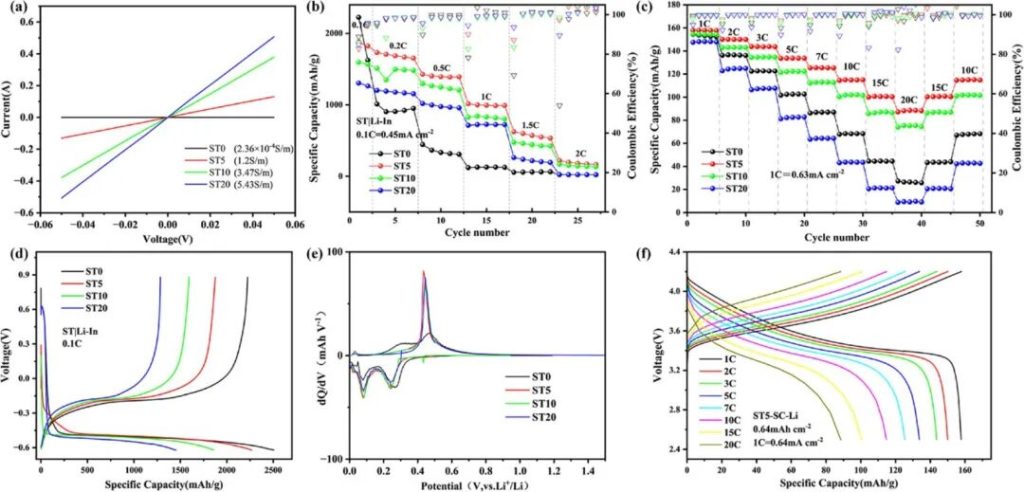
Figure 3 | a) Comparison of electronic conductivity of ST0/5/10/20; b) Rate performance of ST/LPSCI/LiIn half-cell; c) Rate performance of LCO/LPSCI/ST-SC-Li full cell; d) Voltage-specific capacity curve of ST0/5/10/20 half-cell at 0.1C; e) dQ/dV curve of ST0/5/10/20 half-cell at 0.1C during the second cycle; f) Voltage-specific capacity curve of ST5-SC-Li full cell at different rates.
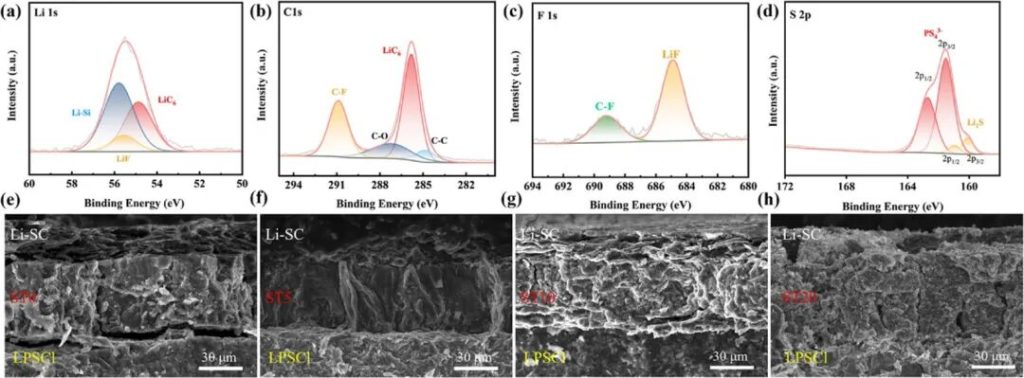
Figure 4 | XPS spectra of ST5-SC-Li anode after cycling: a) Li 1s, b) C 1s, c) F 1s, d) S 2p. e–h): SEM images of the cross-section of ST0/5/10/20 SC-Li anode after cycling.
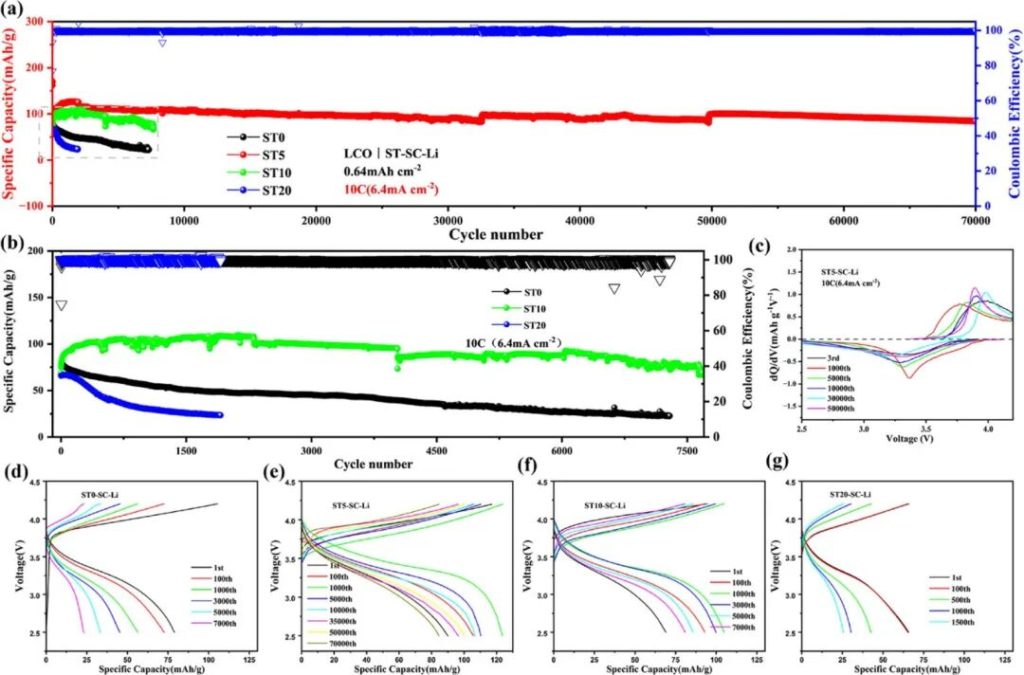
Figure 5 | a) Cyclic performance of LCO/LPSCI/ST-SC-Li full cells; b) Magnified view during cycle testing; c) dQ/dV curves of ST5-SC-Li cells at different cycle numbers; d–g) Voltage-specific capacity curves of ST0/5/10/20-SC-Li cells at different cycle numbers.
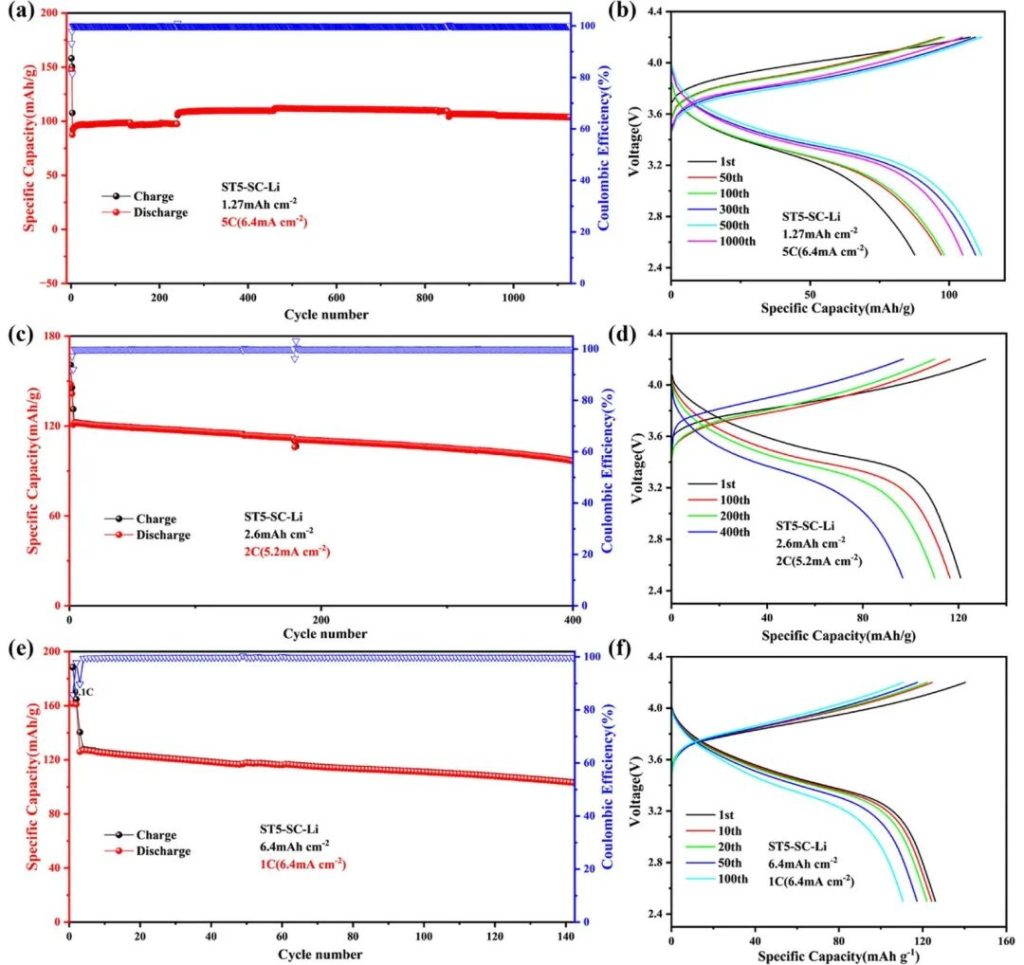
Figure 6 | LCO | ST5-SC-Li full cell, a) Cycling performance at 5C (6.4 mA cm⁻²). b) Voltage-specific capacity curves at different cycle numbers at 5C. c) Cycling performance at 2C (5.2 mA cm⁻²). d) Voltage-specific capacity curves at different cycle numbers at 2C. e) Cycling performance at 1C (6.4 mA cm⁻²). f) Voltage-specific capacity curves at different cycle numbers at 1C.
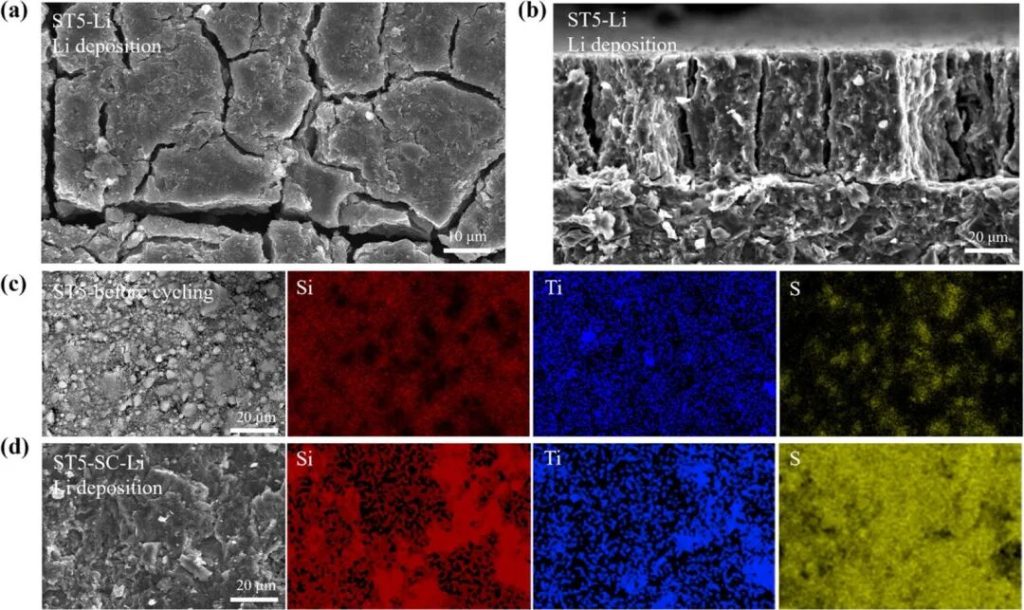
Figure 7 | SEM images of ST5-Li anode after lithium deposition: a) surface and b) cross section; c) SEM and EDS of ST5 sheet before cycling; d) SEM and EDS of ST5-SC-Li surface after cycling.
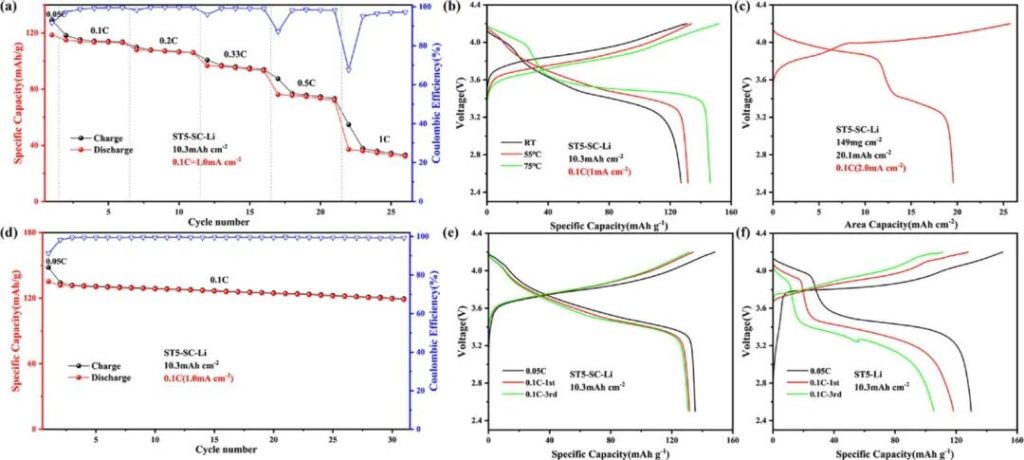
Figure 8 | LCO|ST5-SC-Li high areal capacity full cell, 10.3 mAh cm⁻²: a) Rate performance; b) Cycling at 0.1 C at different temperatures; c) 20.1 mAh cm⁻²: Voltage-areal capacity curve at 0.1 C; 10.3 mAh cm⁻²: d) Cycling performance; e) Voltage-specific capacity curves at 0.05 C and 0.1 C; LCO|ST5-Li (10.3 mAh cm⁻²): f) Voltage-specific capacity curves at 0.05 C and 0.1 C.
Conclusions and Outlook
This study utilizes ball milling and sintering processes to create a highly electronically conductive TiSi₂ network, constructing a three-dimensional rigid support structure encapsulating silicon particles. This structure effectively suppresses silicon volume expansion and provides interfacial pinning. The study combines a sulfide electrolyte (LPSCl) to establish an “ion-electron dual pathway,” introducing a soft carbon interlayer to buffer stress and suppress lithium dendrite growth, while a lithium metal layer achieves dynamic capacity compensation. This multi-layered synergistic design achieves coordinated optimization of the three core issues in silicon-based anodes: volume expansion, lithium dendrite growth, and interfacial instability. An LCO/LPSCl/ST5-SC-Li battery assembled with ST5 material containing 5% Ti exhibits no capacity decay after 64,000 cycles at 10C rate with a 0.64 mAh cm⁻² areal capacity, and a total charge-discharge time exceeding 10,000 hours. Even under high load conditions, this structure remains highly efficient, achieving stable cycling for 30 cycles (capacity retention > 90%) at a 10.3 mAh cm⁻² areal capacity, and achieving an ultra-high reversible areal capacity of 19.6 mAh cm⁻², while also possessing a wide operating temperature range. This research provides an innovative multilayer structure strategy for overcoming the bottlenecks in silicon-based anode applications, contributing to the development of high-energy-density sulfide-based all-solid-state batteries.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
If you do battery research or battery materials research, you might be interested in these: )
