Another “Nature” about battery: A ductile solid electrolyte interphase for solid-state batteries
Solid-state lithium metal batteries are facing huge challenges under practical working conditions. Even when the ionic conductivity of composite solid-state electrolytes is increased to 1 mS cm−1, it is still difficult to realize long-life cycling of solid-state batteries above a current density of 1 mA cm−2 and an areal capacity of 1 mAh cm−2 (ref.). The fundamental cause is the brittle nature of the solid–electrolyte interphase (SEI) with sluggish lithium-ion transport and the resulting lithium dendrites and severe side reactions. Here we report a ductile inorganic-rich SEI that retains its structural integrity while allowing easy ion diffusion at high current densities and areal capacities. The ductility of the SEI is ascribed to the Ag2S and AgF components, which are formed by a substitution reaction between Li2S/LiF in the SEI and AgNO3 in the dielectric composite electrolytes. Even at a high current density of 15 mA cm−2 and an areal capacity of 15 mAh cm−2, a symmetrical lithium cell with such an SEI has a long cycle life of over 4,500 hours. Furthermore, the ductile SEI also works over 7,000 hours at −30 °C, even under practical conditions of 5 mA cm−2 and 5 mAh cm−2.
Solid-state lithium metal batteries are hailed as the next generation of power batteries due to their high energy density and safety, and have broad application prospects in electric vehicles and large-scale energy storage. However, the commercialization of solid-state batteries has long been hampered by challenges such as the low ionic conductivity of solid electrolytes and the stability of the solid-solid interface due to the difference between the solid electrolyte and the electrode. Although numerous studies have significantly improved the ionic conductivity of solid electrolytes, solid-state batteries are prone to interface failure under harsh operating conditions such as high current density and low-temperature charge-discharge. Extensive research has shown that while the traditional inorganic-rich solid electrolyte interface (SEI) on the lithium metal anode surface possesses a high Young’s modulus, its intrinsic brittleness makes it prone to brittle fracture during cycling. This leads to slow lithium-ion transport kinetics and severe lithium dendrite growth and interfacial side reactions, making it difficult for solid-state batteries to achieve long-life stable cycling at low temperatures and high current densities. This problem remains unsolved.
Recently, a team led by Professor Kang Feiyu, Professor He Yanbing, Associate Professor Lü Wei, and Assistant Professor Hou Tingzheng from the Institute of Materials Science and Engineering at Tsinghua University’s Shenzhen International Graduate School, in collaboration with Professor Yang Quan Hong’s team from Tianjin University, conducted systematic research. They innovatively proposed the design concept of a “plastic inorganic-rich SEI” and developed a novel plastic SEI that combines excellent mechanical properties, lithium-ion transport performance, and gradient lithium-affinity/lithium-repellent characteristics, significantly improving the cycle stability of solid-state batteries at high current densities and low temperatures.
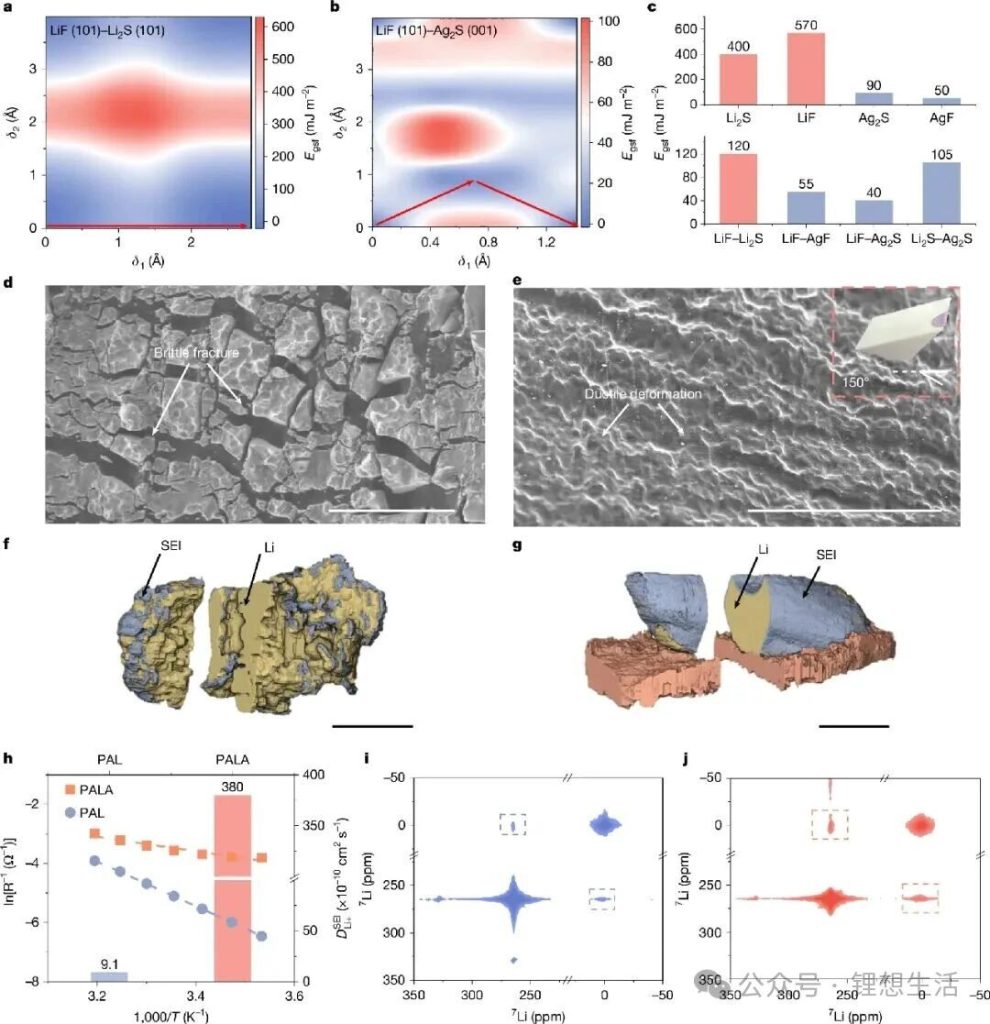
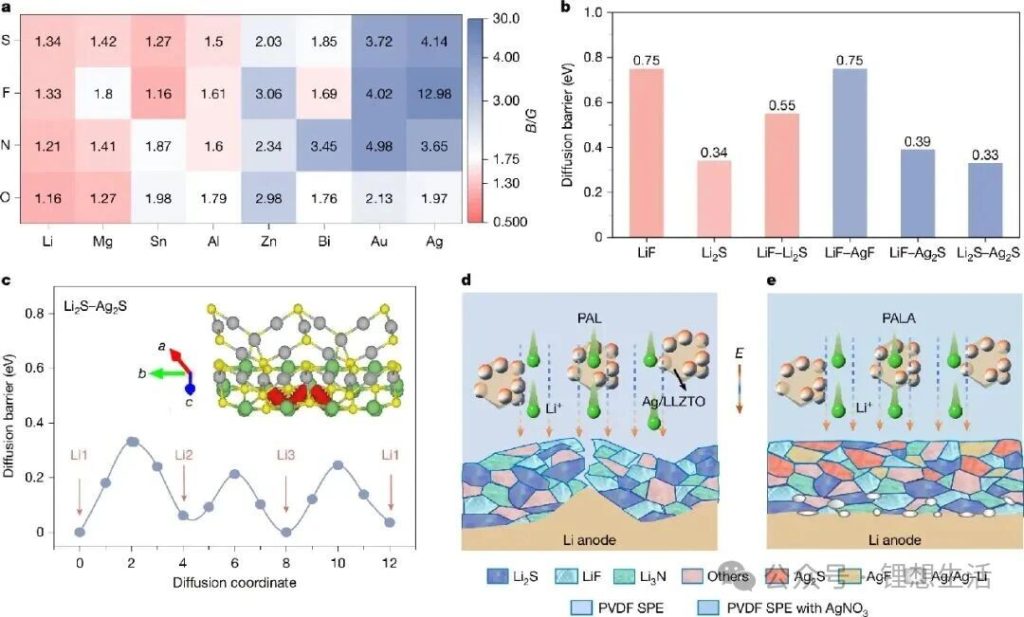
The research team innovated from the ground up in terms of SEI structure and model, abandoning the traditional “strength-only” structural model and design concept for SEIs. They used “plasticity” as the core indicator for screening novel SEI components, employing the Pugh criterion (a B/G ratio ≥ 1.75 indicates that the component has plasticity, where B is the bulk modulus and G is the shear modulus). Through AI-accelerated theoretical screening of a series of potential inorganic materials, they discovered that materials such as silver sulfide and silver fluoride not only possess good plastic deformation capabilities but also significantly reduce the lithium-ion diffusion barrier.
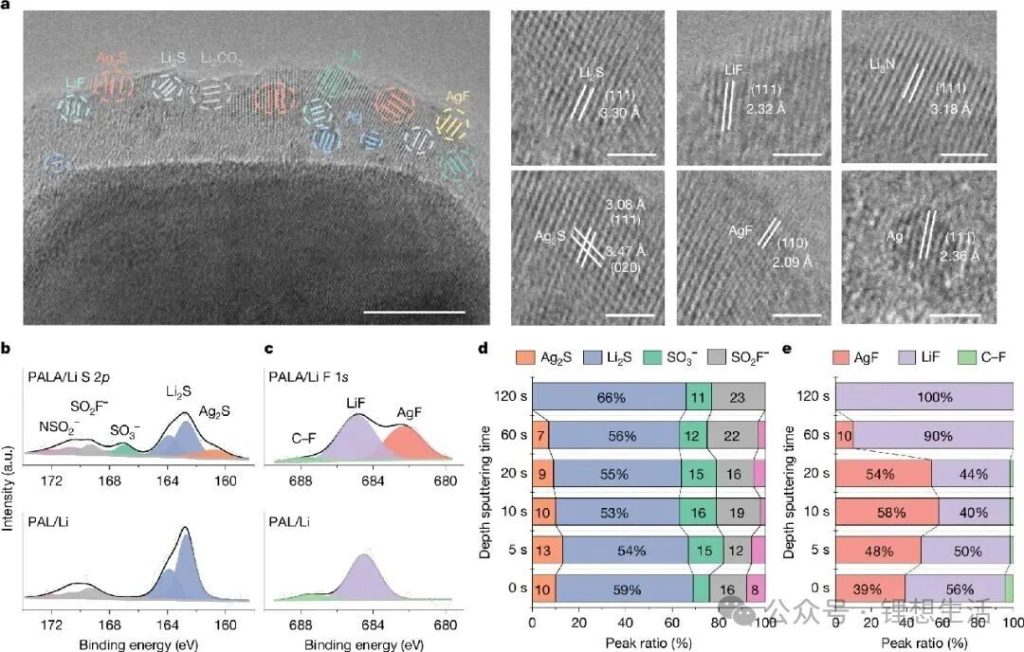
Based on the screening of target components for a plastic SEI, the research team designed an organic/inorganic composite solid-state electrolyte containing AgNO3 additives and Ag/Li6.75La3Zr1.5Ta0.5O12 (LLZTO) filler. This system can transform the brittle Li2S/LiF component into a plastic Ag2S/AgF component through an in-situ substitution reaction during solid-state battery operation, constructing an SEI with a “flexible outer layer and rigid inner layer” gradient structure. In this structure, the outer plastic Ag2S/AgF component dissipates interfacial stress, the middle layer rich in traditional SEI components effectively maintains the traditional high modulus, and the inner lithiophilic component induces uniform lithium metal deposition. This multi-level synergistic design concept of the SEI acts like a “plastic armor” tailor-made for the lithium metal anode, ensuring the structural integrity of the interfacial layer during operation under low temperature and high current density conditions, while also achieving efficient ion transport and suppressing side reactions.
Meanwhile, the Ag/LLZTO ion-electron hybrid conductor ceramic filler modified with Ag particles significantly improved the bulk dielectric properties of the composite solid electrolyte, constructing an efficient lithium-ion transport channel and achieving rapid and uniform lithium-ion deposition. The results show that this “plastic SEI” enables the solid-state battery to exhibit excellent electrochemical performance. At room temperature and with a current density of 15 mA cm⁻² and an areal capacity of 15 mA h cm⁻², the lithium metal symmetric battery can cycle stably for over 4500 hours; at -30°C, the symmetric battery can still cycle stably for over 7000 hours with a current density of 5 mA cm⁻² and an areal capacity of 5 mA h cm⁻²; the solid-state full cell matched with a LiNi₀.₈Co₀.₁Mn₀.₁O₂ cathode also exhibits excellent high-rate (20 C) and low-temperature (-30°C) electrochemical performance.
Test battery performance, use Neware battery cyclers and temp chamber
This work breaks through the traditional SEI design philosophy that focuses on pursuing “rigidity,” innovatively using “ductility” as a characteristic indicator. It proposes a precise construction path from solid-state electrolyte component design to the ideal interface, providing a novel strategy for solving the interface failure problem in solid-state batteries and offering important theoretical basis for the design of novel interface layers. This has significant practical value for the development of practical solid-state batteries.
The related research results, titled “A ductile solid electrolyte interphase for solid-state batteries,” were published online in the journal *Nature*.
The corresponding authors of this paper are Kang Feiyu, He Yanbing, Lü Wei, Hou Tingzheng, and Yang Quan Hong. Mi Jinshuo (PhD student, Class of 2022), Yang Jun (Master’s student, Class of 2023), Chen Likun (PhD student, Class of 2021), and Cui Wenting (PhD student, Class of 2022) from Tsinghua University Shenzhen International Graduate School are co-first authors. Other authors include Associate Professor Gan Lin and Associate Professor Liu Ming from Tsinghua University Shenzhen International Graduate School, Assistant Professor Han Bing from Ningbo Oriental University of Technology, and Associate Professor Huang Yanfei from Shenzhen University. This research was supported by several projects, including the National Natural Science Foundation of China, the National Key Research and Development Program of China (Engineering Science and Interdisciplinary Engineering), the Shenzhen Science and Technology Program, and the Pengrui Start-up Program.
Paper link: A ductile solid electrolyte interphase for solid-state batteries
If you do battery research or battery materials research, you might be interested in these: