Introduction to Cathode Materials for Lithium-ion Batteries
I. Components of a Lithium-ion Battery
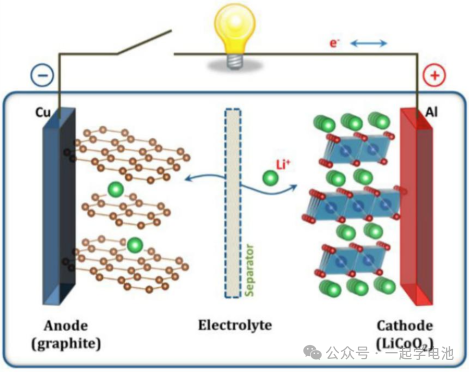
A lithium-ion battery consists of four primary components: the cathode, the anode, the electrolyte, and the separator.
The cathode material is the core component that determines key performance metrics, including energy density, capacity, stability, and safety of the battery.
The performance of the cathode material directly impacts the battery’s cycle life, charge-discharge efficiency, and overall performance across various applications, such as electric vehicles (EVs) and consumer electronics.
Use Neware battery testing system test your batteries
| Battery Category | Energy Density Requirements | Cycle Life Requirements |
| 1. Consumer Batteries | • Specific energy of single cell ≥ 260 Wh/kg • Specific energy of battery pack ≥ 200 Wh/kg • Volumetric energy density of polymer single cell ≥ 650 Wh/L | • Cycle life of single cell and battery pack ≥ 800 cycles, with capacity retention ≥ 80% |
| 2. Power Batteries | ||
| Small Power Batteries | • Specific energy of single cell ≥ 140 Wh/kg • Specific energy of battery pack ≥ 110 Wh/kg | • Single cell cycle life ≥ 1000 cycles, with capacity retention ≥ 70% • Battery pack cycle life ≥ 800 cycles, with capacity retention ≥ 70% |
| Large Power Batteries (Energy-optimized) | • Using ternary materials (NCM/NCA): Single cell specific energy ≥ 230 Wh/kg, battery pack specific energy ≥ 165 Wh/kg • Using other materials (e.g., LFP): Single cell specific energy ≥ 165 Wh/kg, battery pack specific energy ≥ 120 Wh/kg | • Single cell cycle life ≥ 1500 cycles, with capacity retention ≥ 80% • Battery pack cycle life ≥ 1000 cycles, with capacity retention ≥ 80% |
| Large Power Batteries (Power-optimized) | • Power density of single cell ≥ 1500 W/kg • Power density of battery pack ≥ 1200 W/kg | |
| 3. Energy Storage Batteries | • Specific energy of single cell ≥ 155 Wh/kg • Specific energy of battery pack ≥ 110 Wh/kg | • Single cell cycle life ≥ 6000 cycles, with capacity retention ≥ 80% • Battery pack cycle life ≥ 5000 cycles, with capacity retention ≥ 80% |
Data Source: Specification Conditions for the Lithium-ion Battery Industry (2024 Edition)
Electrochemical Performance
Capacity
The amount of electricity that a battery can deliver under specific discharge conditions is called the battery capacity, represented by the symbol $C$. The commonly used unit is the Ampere-hour, abbreviated as Ah, or Milliampere-hour (mAh).
Battery capacity can be categorized into theoretical capacity, rated capacity, and actual capacity. ————-> Neware battery tester
Theoretical capacity is the maximum theoretical value calculated from the mass of the active material according to Faraday’s law. To compare different battery series, the concept of specific capacity is often used, which refers to the theoretical amount of electricity delivered per unit volume or unit mass of the battery. The units are Ah/kg (mAh/g) or Ah/L (mAh/cm³).
Actual capacity refers to the amount of electricity a battery can output under specific conditions. It is equal to the product of the discharge current and the discharge time, measured in Ah. Its value is lower than the theoretical capacity.
Rated capacity, also known as guaranteed capacity, is the minimum capacity that a battery is guaranteed to discharge under specific discharge conditions, according to standards issued by the state or relevant authorities.
II. Classification of Cathode Materials
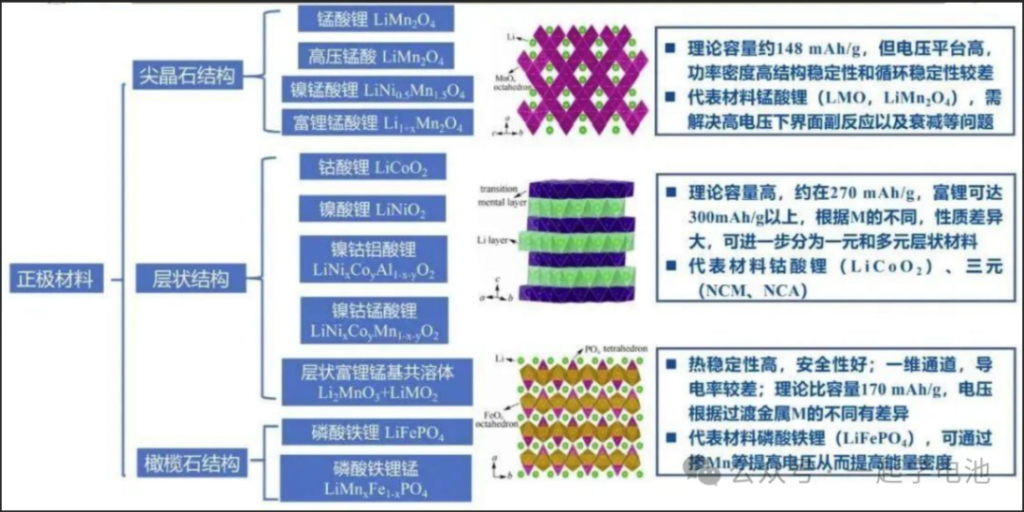
1. Spinel Structure
Main Materials:
Lithium Manganese Oxide
High-voltage Lithium Manganese Oxide
Lithium Nickel Manganese Oxide
Lithium-rich Manganese-based Oxide
Key Characteristics:
Theoretical capacity is approximately 148 mAh/g, but features a high voltage platform and high power density. Structural stability and cyclic stability are relatively poor.
Representative Material: Lithium Manganese Oxide (LMO). Key challenges involve resolving interface side reactions at high voltage and capacity fading.
2. Layered Structure
Main Materials:
Lithium Cobalt Oxide
Lithium Nickel Oxide
Lithium Nickel Cobalt Aluminum Oxide
Lithium Nickel Cobalt Manganese Oxide
Layered Lithium-rich Manganese-based Solid Solution
Key Characteristics:
High theoretical capacity, approximately 270 mAh/g; lithium-rich versions can exceed 300 mAh/g. Properties vary significantly based on the metal composition. It can be further categorized into single-metal and multi-metal layered materials.
Representative Materials: Lithium Cobalt Oxide (LCO) and Ternary materials (NCM, NCA).
3. Olivine Structure
Main Materials:
Lithium Iron Phosphate
Lithium Manganese Iron Phosphate
Key Characteristics:
High thermal stability and excellent safety. It features one-dimensional channels, though electrical conductivity is relatively poor. Theoretical specific capacity is 170 mAh/g, with voltage variations depending on the transition metal.
Representative Material: Lithium Iron Phosphate (LFP). Energy density can be improved by increasing the voltage through Manganese-doping (Mn-doping).
1. Spinel Structure
Representative Material: Lithium Manganese Oxide
Common Derivatives: High-voltage Lithium Manganese Oxide, Lithium Nickel Manganese Oxide, Lithium-rich Manganese-based Oxide
Core Characteristics:
Theoretical Capacity: Approximately 148 mAh/g.
Advantages: High voltage platform and high power density.
Disadvantages: Relatively poor structural and cyclic stability.
2. Layered Structure
Representative Materials: Lithium Cobalt Oxide, Ternary Materials, etc.
Main Materials: Lithium Cobalt Oxide, Lithium Nickel Oxide, Lithium Nickel Cobalt Manganese Oxide (NCM), Lithium Nickel Cobalt Aluminum Oxide (NCA), Layered Lithium-rich Manganese-based Solid Solution
Core Characteristics:
Theoretical Capacity: Generally high, approximately 270 mAh/g. Lithium-rich materials can exceed 300 mAh/g.
Advantages: High theoretical capacity.
Note: Material properties vary significantly depending on the transition metal (M). These can be further categorized into single-metal and multi-metal layered materials.
3. Olivine Structure
Representative Material: Lithium Iron Phosphate
Main Materials: Lithium Iron Phosphate (LFP), Lithium Manganese Iron Phosphate (LMFP)
Core Characteristics:
Theoretical Capacity: Approximately 170 mAh/g.
Advantages: High thermal stability and excellent safety.
Disadvantages: Lithium-ion diffusion occurs through one-dimensional channels; intrinsic electronic and ionic conductivity are relatively poor.
Note: The voltage platform varies depending on the transition metal (M). Voltage can be increased through Manganese (Mn) doping to improve energy density.
III. Common Issues and Solutions about Cathode Materials
1. Specific Capacity Below Standards
Sometimes, the specific capacity of cathode materials is lower than expected, failing to meet the energy density required by the battery design. This is typically related to material quality, processing techniques, and formulation.
Solutions:
Optimize Material Formulation: Adjust the synthesis process of cathode materials, such as modifying the nickel, cobalt, and manganese ratios in ternary materials or adopting higher-performance materials.
Surface Modification: Apply surface coatings or modifications to the materials to improve the structural stability of the electrode materials.
2. Poor Uniformity
Poor coating uniformity of electrode materials can lead to increased internal resistance, low energy conversion efficiency, and even issues like short circuits or thermal runaway.
Solutions:
Optimize Coating Process: Utilize high-precision coating equipment to ensure uniform coating thickness.
Adjust Slurry Viscosity and Composition: Rationally adjust the slurry formulation based on the characteristics of different materials to improve fluidity and coatability.
3. Non-uniform Drying
Non-uniform drying after electrode coating leads to stress differentials within the material’s internal structure, which compromises battery performance. Excessive drying speeds may cause uneven coating layers, while excessively high temperatures can damage battery materials.
Solutions:
Optimize Drying Process: Control drying temperatures and durations to avoid speeds that are too fast or too slow, ensuring the coated material dries uniformly.
Uniform Hot Air Distribution: Utilize uniform hot air systems to ensure even heating during the drying process, preventing material delamination or deformation.
4. Suboptimal Porosity and Density
Inappropriate electrode porosity or density results in poor conductivity and high internal resistance, adversely affecting battery performance.
Solutions:
Optimize Slurry Composition and Coating Methods: Adjust slurry viscosity and material ratios to optimize the porosity of the electrode material.
Control Compaction Process: Implement appropriate compaction (calendering) processes to ensure the electrode material reaches the correct density, avoiding the degradation of conductivity caused by excessive porosity.
IV. Summary and Feedback
1. What are ternary materials (NCM/NCA), and what are their constituent elements?
Ternary materials (NCM/NCA) are lithium-ion battery cathode materials composed of Nickel (Ni), Cobalt (Co), and Manganese (Mn), or Nickel (Ni), Cobalt (Co), and Aluminum (Al). They are characterized by high energy density and are widely used in fields with high energy requirements, such as electric vehicles and consumer electronics.
2. What are the advantages of Lithium Iron Phosphate (LFP) cathode materials?
High Safety: Compared to other cathode materials, LFP possesses higher thermal and chemical stability, effectively preventing issues such as overheating and thermal runaway.
Long Cycle Life: LFP typically has a long cycle life and can withstand a higher number of charge-discharge cycles.
Lower Cost: The raw materials for Lithium Iron Phosphate are abundant and low-cost, which helps reduce the overall cost of the battery.
Environmentally Friendly: LFP materials are non-toxic, non-polluting, and relatively environmentally friendly.
3. What is the basis for choosing between Lithium Nickel Cobalt Manganese Oxide (NCM) and Lithium Iron Phosphate (LFP) in electric vehicle applications?
Energy Density Requirements: NCM has a higher energy density, making it suitable for electric vehicles requiring longer range, especially in the high-end market. LFP has a relatively lower energy density, making it suitable for short-range or city vehicles.
Cost Considerations: LFP is lower in cost and resource-abundant, making it suitable for cost-sensitive markets, particularly mid-to-low-end electric vehicles. NCM has a higher cost due to its cobalt content.
Safety Requirements: LFP offers higher safety and remains more stable under high temperatures or overcharge conditions, making it ideal for applications requiring high safety standards. NCM is relatively more susceptible to the effects of overcharging or high temperatures.
Cycle Life: LFP materials have a longer cycle life, making them suitable for vehicles with high battery longevity requirements.
4. What are the solutions if poor slurry coating uniformity occurs during the electrode manufacturing process?
Adjust the slurry formulation to improve viscosity; optimize the coating process; and optimize the drying process. Additionally, strictly control moisture levels and temperature.
5. Why is choosing the appropriate drying temperature and speed critical?
Excessive drying speeds can lead to non-uniform coatings, compromising battery performance. Meanwhile, excessively high temperatures may cause damage to the battery materials.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.