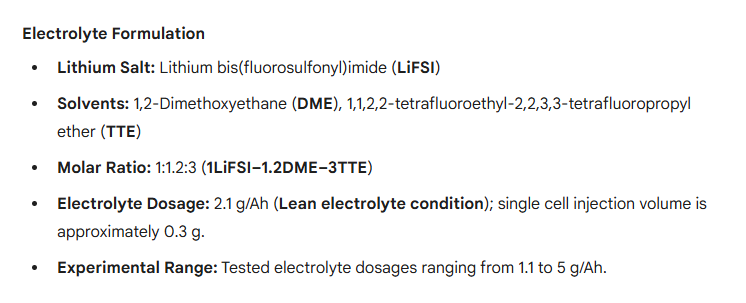
On April 21, 2025, at its Super Technology Day, CATL officially unveiled its “Self-Generated Anode” (SGA) technology. This innovation eliminates the use of traditional materials like graphite as the anode. Instead, by precisely controlling the deposition process of metallic elements (such as Lithium or Sodium), it forms a uniform and dense metallic layer directly on the current collector surface, significantly boosting the battery’s energy density.
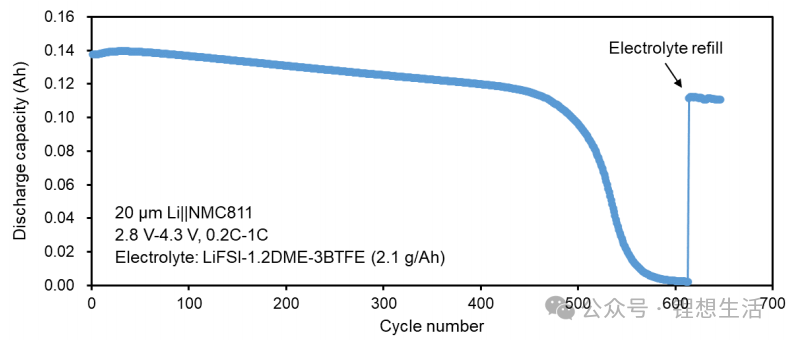
Through nanoscale interface structural design, the technology optimizes ion conduction paths, ensuring stable metal deposition during charge and discharge cycles while minimizing side reactions and active ion loss. This breakthrough addresses the long-standing challenge of cyclic degradation in lithium-metal anodes. Consequently, the ion conduction rate has increased 100-fold, active ion consumption has been reduced by 90%, and storage performance has improved by 300%.
The technology achieves a 60% increase in volumetric energy density and a 50% increase in gravimetric energy density. It can be flexibly applied across various chemical systems, including Sodium-ion (Na-ion), LFP, and NCM (Ternary), transcending the performance constraints of single materials. Its performance when paired with different cathodes is remarkable:
Sodium-ion System: 350 Wh/L
LFP System: 680–780 Wh/L
Ternary (NCM) System: Exceeding 1000 Wh/L
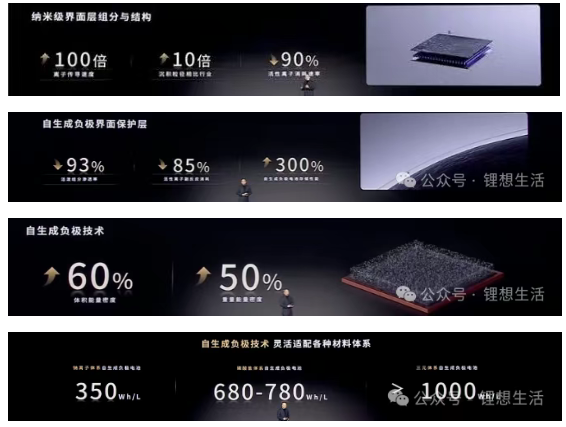
According to the research paper titled “Application-driven design of non-aqueous electrolyte solutions through quantification of interfacial reactions in lithium metal batteries” published by the CATL research team in Nature Nanotechnology, the design details of CATL’s self-generated anode battery (also known as the anode-free battery) are summarized as follows:
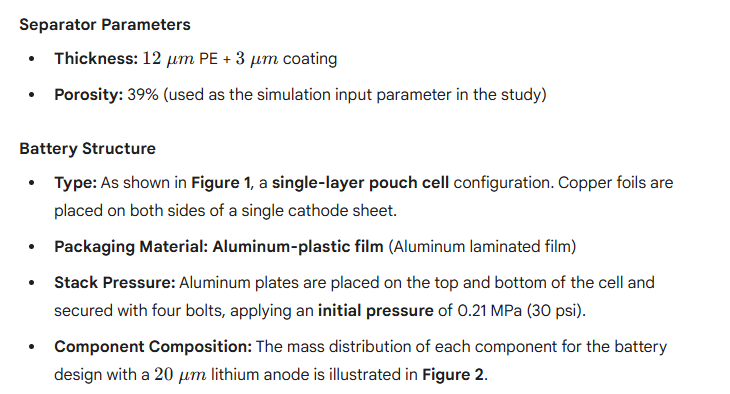
The anode-free battery utilizes copper foil as the anode current collector substrate, with no lithium metal or active materials pre-loaded in its initial state. During the initial charge, lithium ions deintercalate from the NMC811 cathode and deposit onto the copper surface to form metallic lithium, which serves as the active lithium source for subsequent cycles. The battery design schematic is illustrated in Figure 1.


CATL’s Anode-Free Battery Performance
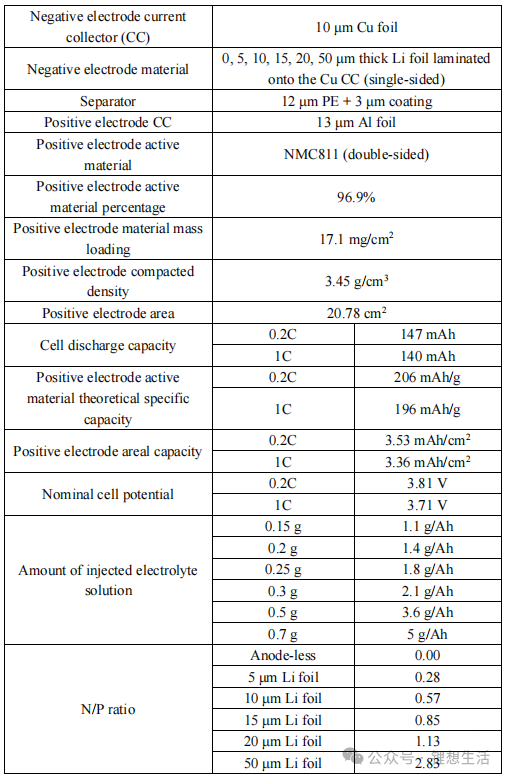
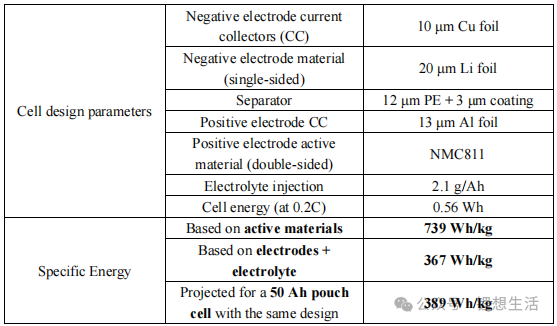
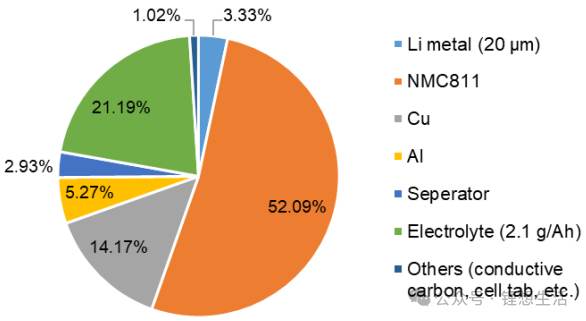
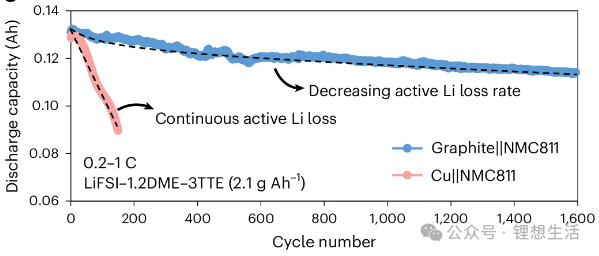
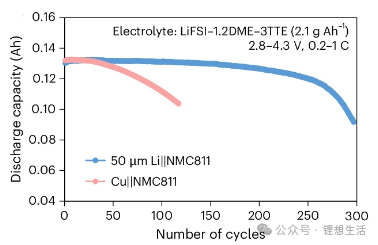
The actual measured discharge capacities of the battery are 147 mAh (at 0.2C) and 140 mAh (at 1.0C), as illustrated in Figures 3 and 4. Under practical conditions—specifically with lean electrolyte 2.1 g Ah⁻¹ and high-loading NMC811 17.1 mg cm⁻²—the anode-free battery sustains only approximately 120 cycles (to 80% capacity retention) with a linear decay profile.
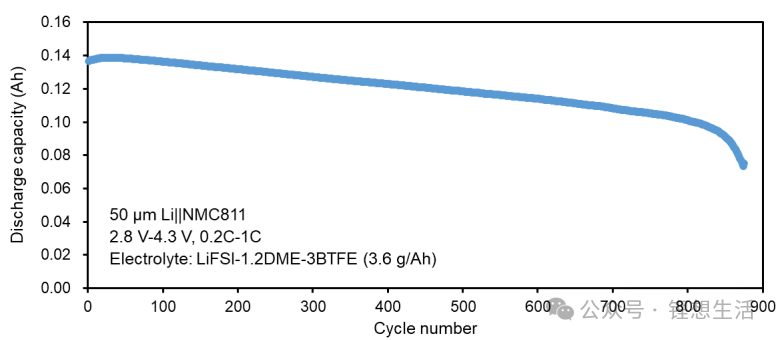
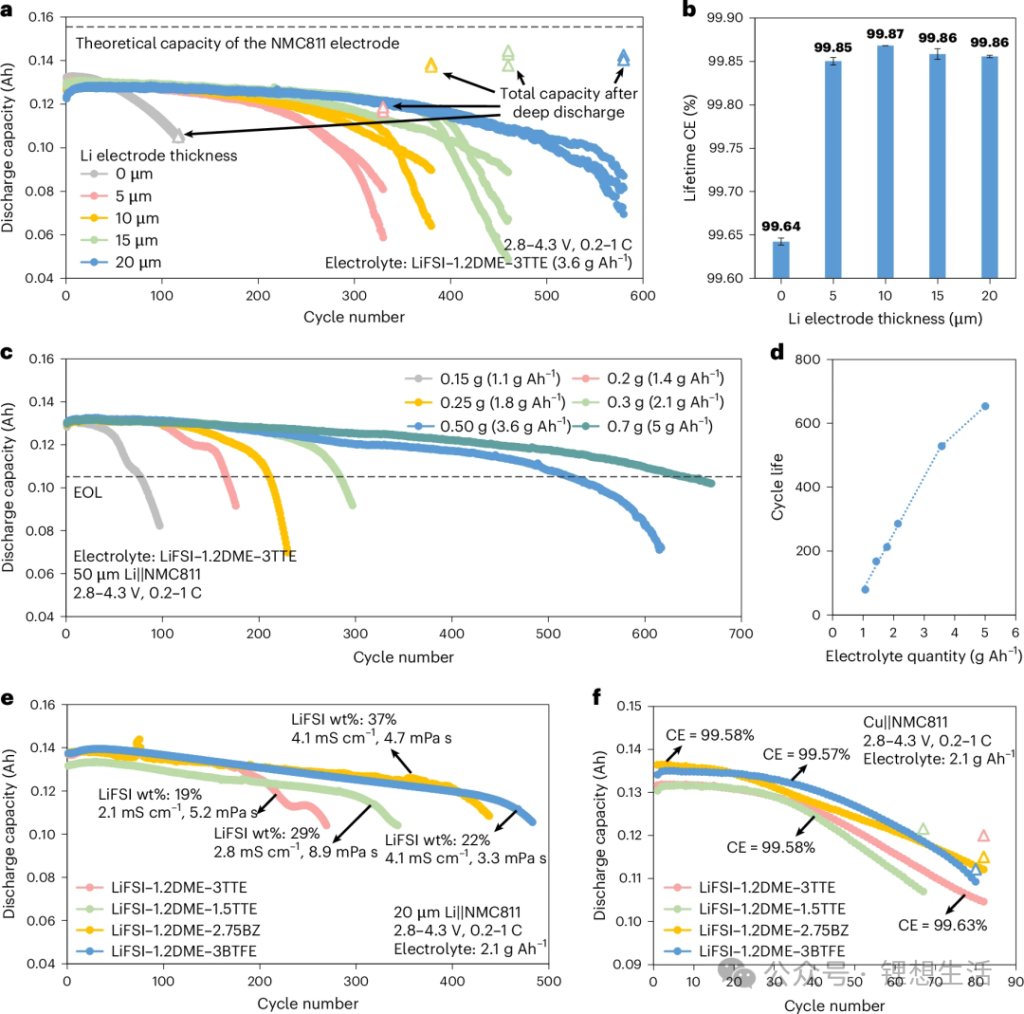
Battery performance can be enhanced by either pre-loading lithium metal on the anode or increasing the electrolyte dosage. As shown in Figure 6:
Effect of Lithium Thickness: With a fixed electrolyte dosage of 3.6 g Ah⁻¹ (flooded conditions to eliminate electrolyte bottlenecks), increasing the lithium metal thickness from 0 μm (pure Cu) to 5, 10, 15, and 20 μm results in a linear extension of cycle life from ~100 cycles to ≈ 600 cycles (for 20 μm). On average, every 1 μm of additional lithium extends the lifespan by approximately 25–30 cycles (at 80% retention).
Effect of Electrolyte Dosage: With a fixed 50 μm lithium foil (ensuring excessive lithium supply), varying the electrolyte dosage from 1.1, 1.4, 1.8, 2.1, 3.6, to 5 g Ah⁻¹ causes the cycle life to rise, reaching 650 cycles at the 5 g Ah⁻¹ level.
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, or EV driving simulation test, please feel free to contact us.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.