Graphite Anodes Explained: Chemical vs. Electrochemical Lithiation in Lithium-Ion Batteries
Source: WeChat Official Account “Brother Radish” 萝卜大师兄
Graphite anode materials have maintained a dominant position in the lithium-ion battery industry since their discovery. While cathode options have diversified significantly—ranging from Lithium Iron Phosphate (LFP) and Lithium Cobalt Oxide (LCO) to NCM Ternary cathodes—graphite remains the undisputed first choice for the anode. But why is this the case? Join us as we trace the developmental history of the graphite anode and explore how it established its long-standing “dynasty” in the battery world.
I. The Discovery of Chemical Lithiation in Graphite (1950s)
In 1955, the French chemist A. Hérold immersed graphite into molten lithium metal, unexpectedly giving birth to a shimmering, golden-yellow compound: the Lithium-Graphite Intercalation Compound (Li-GIC). This discovery provided the first proof that lithium ions (Li+) could be intercalated into the layered structure of graphite.
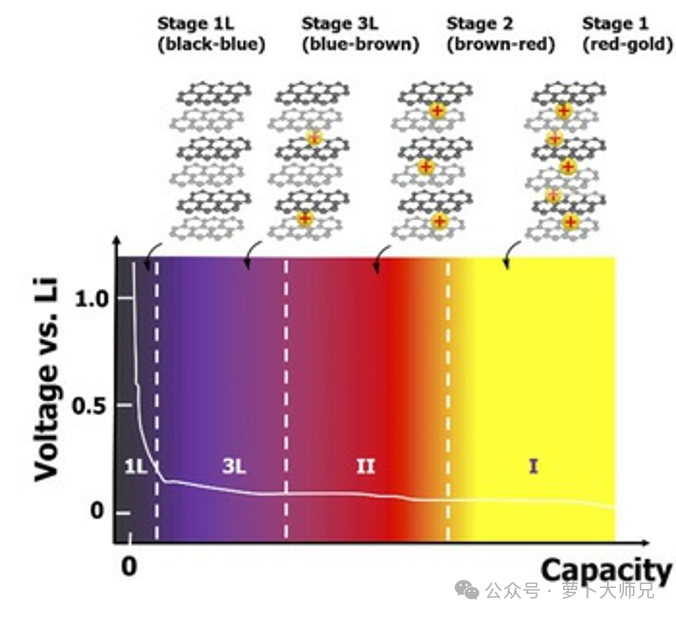
Subsequent research revealed an even more sophisticated “Staging Structure” during the lithiation process:
Ordered Intercalation: Lithium ions do not insert randomly or uniformly; instead, they form ordered structures with specific stoichiometric ratios.
Staged Transitions: As the lithium content increases, the compound undergoes various “stages” (e.g., Stage III, Stage II).
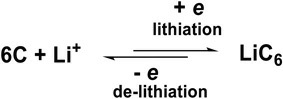
Theoretical Capacity: The process culminates in the Stage I structure (LiC6), where one lithium atom corresponds to six carbon atoms. This yields a theoretical mass specific capacity of 372 mAh/g (or 340 mAh/g if calculated as LiC6) and a volumetric capacity of approximately 850 mAh/cm³.
However, the chemical method of lithiation in graphite faced several fatal limitations:
Harsh Conditions: The process requires molten lithium (>180°C) or lithium vapor (low-temperature vacuum environments).
Irreversibility: It is essentially the unidirectional diffusion of lithium atoms (Li0) into the graphite lattice, making extraction extremely difficult.
Non-Electrochemical Nature: This process is fundamentally different from the ionic intercalation/de-intercalation mechanism required for battery operation.
II. The Dilemma of Electrochemical Lithiation in Graphite (Pre-1990)
The question then arose: could LiC6 be used directly as a pre-formed graphite anode? This idea was proposed by researchers well before 1990. However, even when LiC6 was synthesized externally and then integrated into a battery as an anode, scientists were unable to achieve efficient and reversible cycling. The core challenges were:
The Reversibility Challenge: How can Li+ be electrochemically “pulled out” from LiC6 while allowing the graphite structure to return to a stable state? Furthermore, how can Li+ be electrochemically intercalated into the layered graphite structure without causing structural damage?
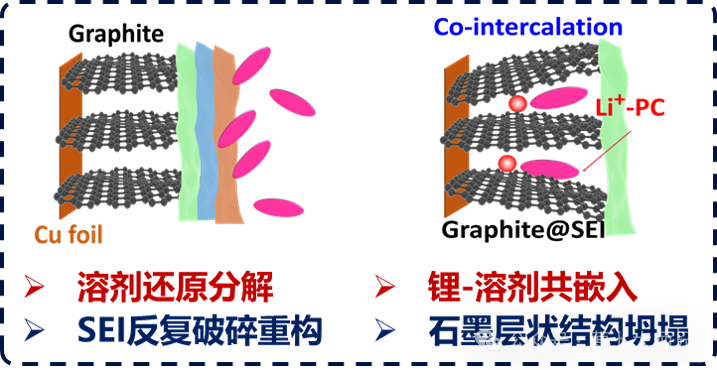
Electrolyte Breakdown: Common organic electrolytes decompose violently at the extremely low lithiation potential of graphite (which is close to that of metallic lithium). This prevents the formation of a stable interface and leads to the persistent problem of continuous solvent decomposition.
Solvent Co-intercalation: The mainstream Propylene Carbonate (PC) based electrolytes of that era underwent co-intercalation at the graphite interface, making reversible lithium intercalation/de-intercalation impossible. Other solvents, such as ethers and sulfones, faced similar issues, resulting in a severe lack of suitable electrolyte solvent options.
Practical Operational Difficulties: Preparing LiC6 chemically outside the cell and then integrating it into a battery as an anode proved extremely difficult. Due to the ultra-high reactivity of LiC6, implementation was nearly impossible. This series of problems led the academic community at the time to assert that graphite could never become a practical anode material for lithium-ion batteries.
III. Breakthrough in Electrochemical Lithiation (The 1990s)
The turning point emerged from a revolutionary discovery in electrolyte chemistry. The critical breakthrough was finding a solvent system capable of forming a stable, dense, and ionically conductive Solid Electrolyte Interphase (SEI film) on the graphite surface. This led to the establishment of Ethylene Carbonate (EC)-based electrolytes (the fundamental principles of which can be referenced in our previous posts):
Controlled Reduction: EC undergoes controlled decomposition at a voltage slightly higher than the lithium intercalation potential, forming a passivating SEI film.
Ionic Channels: The SEI film acts as a molecular sieve, allowing Li+ to pass through while effectively blocking solvent molecules from co-intercalating and destroying the graphite structure.
Reversible Cycling: Protected by the SEI film, Li+ is able to intercalate and de-intercalate between graphite layers reversibly for thousands of cycles.
This is a highly reversible single-electron redox reaction.
IV. The Overwhelming Advantages of Graphite Anodes
Once the electrolyte barrier was broken, the inherent natural advantages of graphite as an anode were immediately unleashed, shining with overwhelming brilliance:
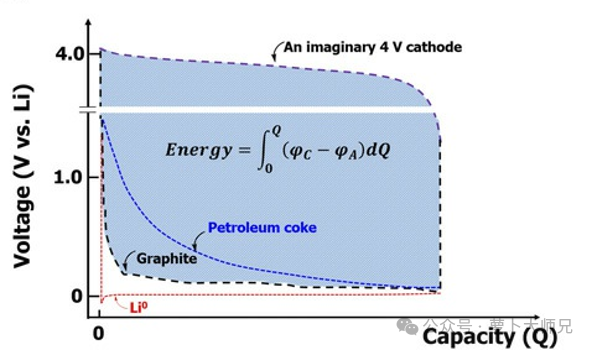
Ultra-low and Flat Voltage Plateau: The lithiation potential remains stable at approximately 0.1 V (vs. Li⁺/Li), which is extremely close to the theoretical minimum of metallic lithium (0 V). The highly ordered crystal structure allows lithium intercalation and de-intercalation to occur at a nearly constant voltage, creating long and flat charge-discharge plateaus. This is a significant advantage over the sloping voltage curves typical of amorphous carbon materials.
Superior Energy Density: The energy density (E) of a battery is calculated by the formula:
E = ∫(U_cathode – U_anode) dQ(Where U represents voltage and Q represents capacity)
Low Voltage (Small U_anode): Directly increases the potential difference relative to the cathode (U_cathode – U_anode).
High Specific Capacity (372 mAh/g): Provides a large amount of transferable charge (dQ).
Flat Plateau: Ensures that the maximum voltage difference is maintained throughout the primary discharge range.
The combination of these factors gives graphite anodes a crushing advantage in energy density, as the area enclosed between the cathode and anode voltage curves is maximized.
Excellent Rate Capability and Cycle Life: The highly ordered layered structure provides rapid diffusion channels for Li+. When combined with a stable SEI film, this ensures high rate performance and a long operational lifespan. Test cycle life—>Neware battery cyclers
V. Establishment of Graphite’s Dominance (Post-1995)
The combination of the electrolyte breakthrough and graphite’s inherent advantages completely reshaped the lithium-ion landscape:
1991: Sony launched the world’s first commercial lithium-ion battery, which initially utilized a petroleum coke (a type of soft carbon) anode.
Circa 1993: Stabilized graphite anode technology based on EC electrolytes reached maturity.
Post-1995: Leveraging its significantly higher energy density and mature mass-production processes, graphite rapidly replaced petroleum coke to become the absolute mainstream anode material. This marked the beginning of the “Graphite Dynasty,” which has dominated the lithium-ion anode market for nearly 30 years.
VI. Future Challenges and Opportunities
Despite constant challenges from emerging materials like silicon-based anodes, graphite remains the backbone of the industry for the foreseeable future due to its perfect balance of cost, process maturity, and overall performance.
The extraordinary journey of graphite lithiation demonstrates that the “impossibilities” hidden within fundamental science are often just waiting for the right moment to be decoded. As we enjoy our lightweight and powerful electronic devices, we should remember this silent “dance of lithium ions” occurring between the layers of graphite—it is the very foundation of modern mobile energy.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.