Introduction to lithium-sulfur battery and lithium-sulfur electrolyte
Source: WeChat Official Account “Learn Batteries Together” 来源于微信公众号 一起学电池
Lithium-sulfur battery (Li-S battery) is a type of lithium battery that uses sulfur as the positive electrode (elemental sulfur is abundant, inexpensive, and environmentally friendly) and metallic lithium as the negative electrode. Due to its high energy density and low-cost raw materials, it is considered a potential candidate for next-generation high-performance batteries. The electrolyte, as a crucial component of lithium-sulfur batteries, directly affects the battery’s performance and lifespan.
Note: The positive electrode material of lithium-sulfur batteries is generally composed of sulfur and a highly conductive material (sulfur itself is non-conductive, so a conductive agent, and a highly conductive one at that, must be added).
01 Charge and Discharge Principles of Lithium-Sulfur Batteries
Typical lithium-sulfur batteries generally use elemental sulfur as the cathode and metallic lithium foil as the anode. Its reaction mechanism differs from the ion intercalation/de-intercalation mechanism of lithium-ion batteries, as it follows an electrochemical mechanism.
In a lithium-sulfur battery, sulfur serves as the cathode active material and lithium as the anode. During discharge, the anode reaction involves lithium losing electrons to become lithium ions. The cathode reaction involves sulfur reacting with lithium ions and electrons to form sulfides. The potential difference between the cathode and anode reactions constitutes the discharge voltage provided by the lithium-sulfur battery. Under an applied voltage, the cathode and anode reactions of the lithium-sulfur battery proceed in reverse, which is the charging process. Based on the amount of electricity provided when a unit mass of elemental sulfur is completely converted to S2-, the theoretical specific discharge capacity of sulfur is 1675 mAh/g. Similarly, the theoretical specific discharge capacity of elemental lithium is 3860 mAh/g. The theoretical discharge voltage of a lithium-sulfur battery is 2.287V when sulfur and lithium react completely to form lithium sulfide (Li2S). Correspondingly, the theoretical specific energy density of the lithium-sulfur battery is 2600 Wh/kg.
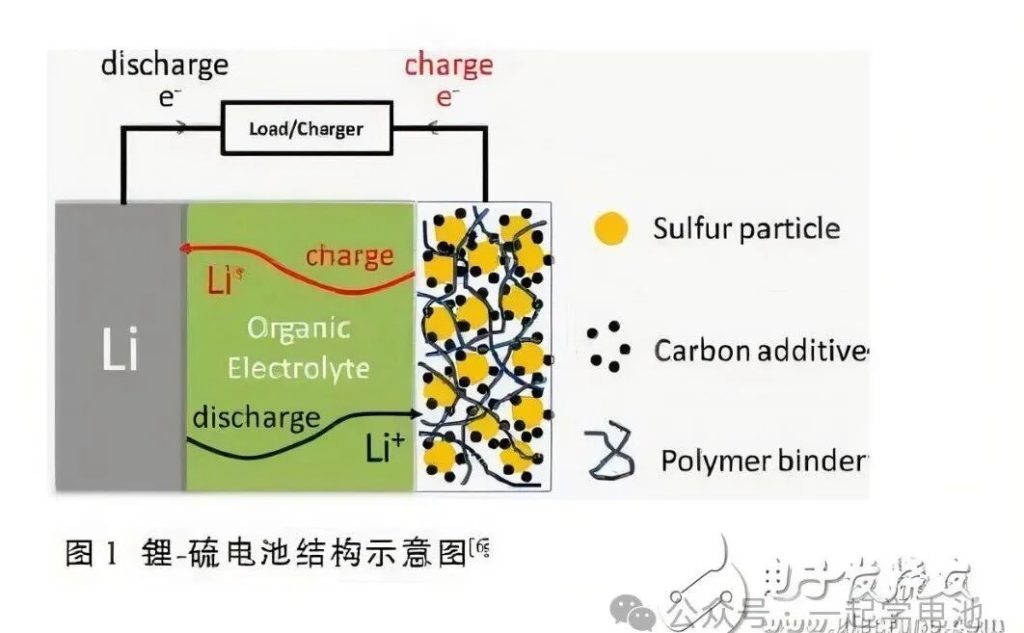
Note: Since sulfur cathodes and lithium anodes can encounter unexpected issues when lithium and electrolyte are in excess, only a few reports on practical lithium-sulfur pouch batteries are available.
02 Lithium-Sulfur Electrolytes
The choice and preparation process of lithium-sulfur electrolytes have a significant impact on battery performance. By rationally selecting solvents, lithium salts, and additives, and by optimizing the preparation process, the energy density, power performance, and cycle life of the battery can be significantly improved.
2.1 Composition
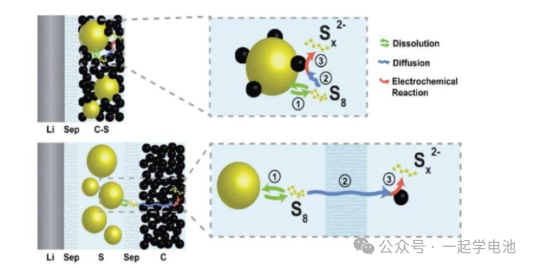
Lithium-sulfur battery electrolytes generally consist of solvents, lithium salts, and additives. During the discharge process, polysulfides (such as Li2S6, Li2S4, etc.) generated at the sulfur cathode dissolve into the electrolyte and may diffuse to the anode, leading to the shuttle effect. This causes capacity loss and self-discharge issues. It is necessary to regulate the dissolution and deposition behavior of polysulfides and suppress the shuttle effect by selecting appropriate electrolyte solvents and additives. The selection and optimization of each component directly affect the performance of the battery.
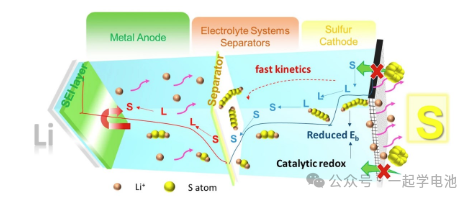
Solvents: Solvents are the primary components of the electrolyte, serving to dissolve lithium salts and provide ion conduction channels. Common solvents include carbonates (such as ethylene carbonate, EC), which possess good electrochemical stability and ionic conductivity but are prone to side reactions in lithium-sulfur batteries. Ethers, such as dimethoxyethane (DME) and diethylene glycol dimethyl ether (DEGDME), are frequently chosen for lithium-sulfur batteries due to their high ionic conductivity and good compatibility. Phosphates (such as triethyl phosphate, TEP) offer high electrochemical stability and flame retardancy, but their ionic conductivity is relatively low.
Lithium Salts: Lithium salts provide lithium ions for the electrolyte. Commonly used lithium salts include lithium hexafluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4), and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). Lithium hexafluorophosphate (LiPF6) has high ionic conductivity but decomposes easily at high temperatures, producing byproducts like HF. Lithium tetrafluoroborate (LiBF4) offers better thermal and electrochemical stability, though its ionic conductivity is slightly lower than that of LiPF6. Lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) features excellent thermal stability and ionic conductivity, but it comes at a higher cost.
Additives: Additives are used to improve electrolyte performance, such as increasing ionic conductivity, enhancing stability, and suppressing side reactions. Common additives include lithium fluoride (LiF) and sulfuryl fluoride (SO2F2). Lithium fluoride (LiF) can form a stable solid electrolyte interface (SEI) on the electrode surface, suppressing the growth of lithium dendrites. Sulfuryl fluoride (SO2F2) has a high redox potential and can serve as an additive for high-voltage electrolytes to improve the energy density of the battery.
2.2 Functions
Ionic Conductivity
Ionic conductivity is a critical metric for evaluating electrolyte performance, directly affecting the battery’s power density and charge/discharge rates. An electrolyte with high ionic conductivity can transport lithium ions more efficiently, reducing internal resistance and improving power output and efficiency. Typically, Electrochemical Impedance Spectroscopy (EIS) is used to measure ionic conductivity, with values ranging from 10-3 S/cm to 10-1 S/cm.
Influencing Factors: Solvent type, lithium salt concentration, and temperature all affect ionic conductivity. Generally, ether-based solvents and high-concentration lithium salts help improve conductivity.
Electrochemical Stability Window
The electrochemical stability window refers to the voltage range within which the electrolyte remains stable and does not undergo decomposition during electrochemical reactions. Since the operating voltage of lithium-sulfur batteries typically ranges from 1.7V to 2.8V, the electrolyte’s stability window must cover this range. Cyclic Voltammetry (CV) is used to determine the decomposition voltage.
Influencing Factors: The types and ratios of solvents and additives affect electrochemical stability. Solvents and additives with high electrochemical stability help expand this window.
Viscosity
Viscosity is a physical property that directly impacts the ion transport capability and cycling performance of the battery. Low-viscosity electrolytes facilitate rapid lithium-ion conduction but may lead to volatilization and leakage; high-viscosity electrolytes can improve stability but may hinder ion transport. Rotational viscometers are typically used for measurement.
Influencing Factors: Solvent type, lithium salt concentration, and temperature influence viscosity. Generally, ether solvents have lower viscosity, while carbonate solvents have higher viscosity.
Temperature Adaptability
Temperature adaptability is evaluated by testing ionic conductivity and electrochemical stability at various temperatures. It represents the electrolyte’s ability to maintain stable performance under different thermal conditions. Because Li-S batteries must operate in diverse environments, the electrolyte needs to maintain good performance across a wide temperature range. The safety of Li-S batteries is heavily influenced by the chemical reactions between polysulfides and the metallic lithium anode. Therefore, selection must prioritize thermal stability and chemical inertness, often involving flame-retardant additives.
Influencing Factors: The boiling and freezing points of solvents, the solubility of lithium salts, and the type of additives all play a role.
Chemical Stability
Chemical stability is assessed through long-term storage and cycling tests, representing the electrolyte’s ability to resist chemical decomposition and side reactions over prolonged use. High chemical stability extends battery life and enhances safety and reliability. ————-Cycling your batteries
Influencing Factors: The purity of solvents and lithium salts, as well as the types and ratios of additives, are key. Using high-purity components and well-matched additives can significantly improve chemical stability.
2.3 Optimization Directions
Promote the formation of a protective layer on the sulfur cathode surface to control the dissolution of polysulfides.
Regulate electrolyte components to control lithium-ion migration and suppress the diffusion of polysulfides (the shuttle effect).
Form a protective layer on the anode surface to inhibit side reactions between polysulfides and the lithium metal.
Solid-state battery design principles primarily focus on improving high interfacial impedance and poor ionic conductivity at room temperature.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
