Lithium-ion battery electrolyte
I. Overview of Lithium-ion Electrolytes
Electrolyte is one of the four key materials of a lithium-ion battery (alongside the cathode, anode, and separator). Often referred to as the “blood” of the battery, it serves the critical role of conducting ions between the positive and negative electrodes. It is the fundamental guarantee for lithium-ion batteries to achieve advantages such as high voltage and high specific energy. Generally, the electrolyte is prepared under specific conditions by mixing high-purity organic solvents, electrolyte lithium salts (such as Lithium Hexafluorophosphate, LiPF6), and necessary additives in precise proportions.
Organic solvents constitute the bulk of the electrolyte and are closely linked to its overall performance. Typically, a mixture of high-dielectric-constant solvents and low-viscosity solvents is used. Common electrolyte lithium salts include Lithium Perchlorate, Lithium Hexafluorophosphate, and Lithium Tetrafluoroborate. However, considering factors such as cost and safety, Lithium Hexafluorophosphate is the primary electrolyte used in commercial lithium-ion batteries. While the use of specific additives has not been fully standardized across all commercial products, it remains one of the most active research hotspots in the field of organic electrolytes.
Since the successful development of lithium-ion battery electrolytes in 1991, lithium-ion batteries quickly entered the market for electronic products such as laptops and mobile phones, gradually gaining a dominant position. Currently, lithium-ion battery electrolyte technology is still undergoing further development. In terms of research and production, international companies engaged in the development of specialized electrolytes are primarily concentrated in Japan, Germany, South Korea, the United States, and Canada. Among them, Japan’s electrolyte industry has developed the fastest and holds the largest market share.
Common electrolyte systems used in China include EC+DMC, EC+DEC, EC+DMC+EMC, and EC+DMC+DEC. Different electrolytes have different operating conditions, compatibility with positive and negative electrodes, and decomposition voltages. An electrolyte composed of Lithium Hexafluorophosphate (LiPF6) mixed with EC, DMC, DEC, and EMC offers better cycle life, low-temperature performance, and safety compared to standard electrolytes. It effectively reduces gas generation and prevents battery swelling. The decomposition voltages for the EC/DEC and EC/DMC electrolyte systems are 4.25V and 5.10V, respectively. According to research, the mixture of LiPF6 with EC and DMC shows excellent compatibility with carbon anodes. For example, in specific battery systems using this electrolyte, it remains stable up to 4.9V at room temperature and 4.8V at 55°C. Its liquid phase range spans from -20°C to 130°C. Its standout advantages include a wide operating temperature range, excellent compatibility with carbon anodes, a high safety index, and superior cycle life and discharge characteristics.
II. Electrolyte Composition
2.1 Organic Solvents
Organic solvents are the primary component of the electrolyte, and its performance is closely related to the properties of these solvents. Commonly used solvents in lithium-ion battery electrolytes include Ethylene Carbonate (EC), Diethyl Carbonate (DEC), Dimethyl Carbonate (DMC), and Ethyl Methyl Carbonate (EMC). Solvents like Propylene Carbonate (PC) and Dimethoxyethane (DME), which are mainly used in primary lithium batteries, are generally avoided. When used in secondary batteries, PC has poor compatibility with the graphite anode. During charging and discharging, PC decomposes on the graphite surface and causes the graphite layers to exfoliate, leading to a decline in cycle performance. However, a stable SEI (Solid Electrolyte Interphase) film can be established in EC or EC+DMC composite electrolytes. It is generally recognized that a mixture of EC and a linear carbonate, such as EC+DMC or EC+DEC, provides excellent electrolyte performance. With the same lithium salt (such as LiPF6 or LiClO4), the PC+DME system consistently shows the worst charge-discharge performance for MCMB (Mesocarbon Microbeads) materials compared to EC+DEC or EC+DMC systems. However, this is not absolute; when PC is used with appropriate additives, it can help improve the low-temperature performance of the battery.
The quality of organic solvents must be strictly controlled before use. For instance, purity must be above 99.9%, and moisture content must be below 10 ppm. There is a close relationship between solvent purity and stable voltage; organic solvents that meet purity standards have an oxidation potential of approximately 5V. This oxidation potential is highly significant for researching overcharge prevention and battery safety. Strictly controlling the moisture in organic solvents is decisive for preparing qualified electrolytes. Reducing moisture below 10 ppm can minimize the decomposition of LiPF6, slow down the breakdown of the SEI film, and prevent battery swelling. Methods such as molecular sieve adsorption, atmospheric or vacuum distillation, and inert gas purging are used to meet moisture requirements.
2.2 Electrolyte Lithium Salts
LiPF6 is the most commonly used electrolyte lithium salt and represents the future direction of lithium salt development. Although LiClO4 and LiAsF6 are used in laboratory settings, LiClO4 is unsuitable for large-scale industrial use because batteries using it perform poorly at high temperatures. Furthermore, LiClO4 is a strong oxidant that can explode upon impact, posing significant safety risks.
LiPF6 is stable toward the negative electrode and offers high discharge capacity, high conductivity, low internal resistance, and fast charge-discharge speeds. However, it is extremely sensitive to moisture and hydrofluoric (HF) acid, reacting easily. Therefore, it must be handled in a dry atmosphere (such as a glove box with moisture levels below 20 ppm). It is also not resistant to high temperatures, decomposing into phosphorus pentafluoride and lithium fluoride between 80°C and 100°C. Due to purification difficulties, it is critical to control self-decomposition caused by the heat released during LiPF6 dissolution and the thermal decomposition of the solvent when preparing the electrolyte. While the purity of domestically produced LiPF6 generally meets standards, the HF acid content is often too high for direct electrolyte preparation and requires further purification. In the past, LiPF6 relied heavily on imports, but several domestic suppliers now provide high-quality products. High-quality LiPF6 ensures that the prepared electrolyte maintains stable moisture and HF levels without becoming viscous or turning red.
2.3 Additives
There is a vast variety of additives available, and different lithium-ion battery manufacturers prioritize different additives based on the specific intended use and performance requirements of their batteries. Generally, the additives used serve three primary functions:
(1) Improving SEI Film Performance Adding anisole or its halogenated derivatives to the lithium-ion battery electrolyte can improve the battery’s cycle performance and reduce irreversible capacity loss. Research into this mechanism has found that anisole reacts with the reduction products of the solvent to generate LiOCH3, which facilitates the formation of a highly efficient and stable SEI film on the electrode surface. A battery’s discharge plateau measures the energy released above 3.6V and reflects the battery’s high-current discharge characteristics to some extent. In practical application, adding anisole to the electrolyte has been shown to extend the discharge plateau and increase the battery’s discharge capacity.
(2) Reducing Trace Water and Hydrofluoric (HF) Acid As previously mentioned, lithium-ion batteries have extremely strict requirements regarding the water and acid content in the electrolyte. Carbodiimide compounds can prevent LiPF6 from hydrolyzing into acid. Additionally, some metal oxides such as Al2O3, MgO, BaO, Li2CO3, and CaCO3 are used to remove HF. However, compared to the rate of LiPF6 hydrolysis, the acid removal speed of these oxides is too slow, and they are difficult to filter out completely.
(3) Preventing Overcharge and Over-discharge Battery manufacturers have an urgent need for improved overcharge and over-discharge resistance. Traditionally, overcharge protection is handled via internal protection circuits. Current research is focusing on adding specific compounds directly to the electrolyte, such as sodium imidazole, biphenyls, and carbazoles. These compounds are currently in the research and development stage.
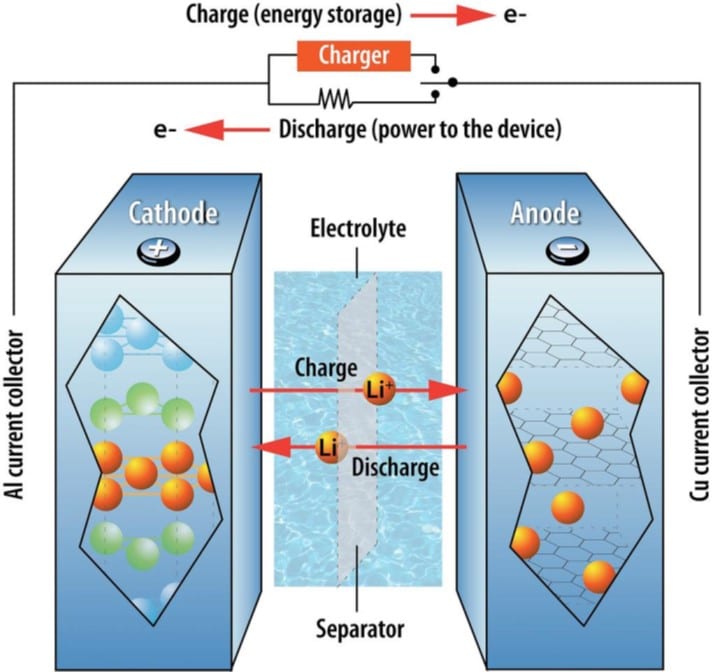
Charging and discharging your batteries
III. Types of Lithium-ion Battery Electrolytes
3.1 Liquid Electrolytes
The choice of electrolyte significantly impacts the performance of lithium-ion batteries. It must possess excellent chemical stability—specifically resisting decomposition at high potentials and temperatures—and maintain high ionic conductivity (> 10⁻³ S/cm). Furthermore, the electrolyte must be inert and non-corrosive to both anode and cathode materials. Since lithium-ion batteries operate at high potentials and utilize chemically active lithium-intercalated anodes, the electrolyte must consist of anhydrous organic compounds rather than water.
Because organic compounds generally exhibit poor ionic conductivity, soluble conductive salts are added. Currently, most lithium-ion batteries use liquid electrolytes with anhydrous organic solvents such as EC, PC, DMC, and DEC, often in mixtures like EC/DMC or PC/DMC. Common conductive salts include LiClO₄, LiPF₆, LiBF₄, and LiAsF₆. Their conductivity levels follow the order: LiAsF₆ > LiPF₆ > LiClO₄ > LiBF₄.
LiClO₄: Due to its high oxidizing nature, it poses explosion hazards and is generally limited to laboratory research.
LiAsF₆: Offers high conductivity and stability but contains toxic arsenic, limiting its use.
LiBF₄: Suffers from poor chemical/thermal stability and lower conductivity.
LiPF₆: Despite its tendency to undergo decomposition reactions, it provides high ionic conductivity. Consequently, it is the industry standard. Most commercial electrolytes utilize LiPF₆ in an EC/DMC mixture for its balanced conductivity and electrochemical stability.
3.2 Solid Electrolytes
Using metallic lithium directly as an anode material offers a very high reversible capacity—theoretically reaching 3,862 mAh/g, which is more than ten times that of graphite. However, it is prone to the growth of lithium dendrites. Solid electrolytes can suppress dendrite growth, making metallic lithium anodes a viable option. Additionally, solid electrolytes eliminate leakage risks, allowing for batteries that are thinner (down to 0.1 mm), higher in energy density, and smaller in volume.
Destructive testing has demonstrated that solid-state batteries are exceptionally safe. While liquid-electrolyte batteries may leak or explode during puncture, heating (200°C), short-circuit, or overcharge (600%) tests, solid-state batteries typically show no safety issues other than a slight rise in internal temperature (< 20°C). Solid polymer electrolytes (SPE) offer flexibility, good film-forming properties, and stability at a low cost, serving as both the separator and the ion-conducting medium.
Solid polymer electrolytes are generally categorized into two types:
Dry Solid Polymer Electrolytes (SPE): These are primarily based on Polyethylene Oxide (PEO). Their main drawback is low ionic conductivity, reaching only 10⁻⁴ S/cm at 100°C. Ion conduction occurs mainly in amorphous regions via the movement of polymer chains. Since PEO’s high molecular regularity leads to crystallization—which hinders ion movement—improving conductivity involves reducing crystallinity and increasing chain mobility or salt solubility. This is achieved through grafting, blocking, cross-linking, or copolymerization.
Gel Polymer Electrolytes (GPE): By adding high-dielectric-constant, low-molecular-weight liquid organic solvents (such as PC) to an SPE, the salt solubility and ionic conductivity are greatly increased. While GPEs exhibit high conductivity at room temperature, they can fail due to “bleeding” (liquid exudation) during use. Gel polymer lithium-ion batteries are currently available as commercial products.
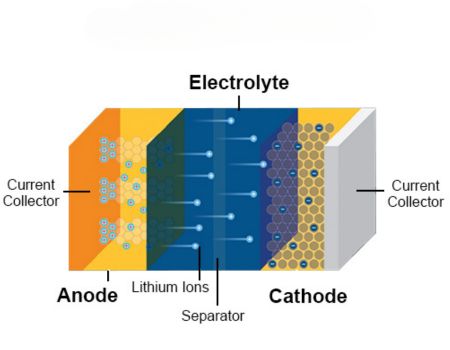
IV. Required Conditions for Lithium-ion Battery Electrolytes
The electrolyte used in lithium-ion batteries is an ionic conductor consisting of lithium salts dissolved in organic solvents. For an organic electrolyte to be practical for commercial use, it must possess the following properties:
High Ionic Conductivity: Generally between 10-3~2*10-3S/cm; the lithium-ion transference number should be close to 1.
Wide Electrochemical Stability Window: It must remain electrochemically stable within a potential range of 0V to 5V.
Excellent Thermal Stability: The electrolyte must remain stable across a wide operating temperature range.
Chemical Stability: It must not react chemically with the current collectors or the active materials inside the battery.
Safety and Low Toxicity: Ideally, the materials should be biodegradable and pose minimal risk.
Solvent Selection
Suitable solvents must have a high dielectric constant and low viscosity. Commonly used alkyl carbonates like PC and EC are highly polar with high dielectric constants; however, their high viscosity and strong intermolecular forces slow down the movement of lithium ions. Conversely, linear esters like DMC (Dimethyl Carbonate) and DEC (Diethyl Carbonate) have low viscosity but also low dielectric constants.
Therefore, to obtain a solution with high ionic conductivity, mixed solvents such as PC+DEC or EC+DMC are typically used. While these organic solvents have a distinct odor, they generally comply with EU RoHS and REACH requirements, making them environmentally friendly materials with very low toxicity.
Conductive Salt Comparison
The primary inorganic anion conductive salts currently developed are LiBF4, LiPF6, and LiAsF6. Their properties are ranked as follows:
Ionic Conductivity: LiAsF6≥LiPF6>LiClO4>LiBF4
Thermal Stability: LiAsF6>LiBF4>LiPF6
Oxidation Resistance: LiAsF6≥LiPF6≥LiBF4>LiClO4
While LiAsF6 offers exceptional conductivity, stability, and charge-discharge rates, its application is strictly limited due to the toxicity of arsenic. Consequently, LiPF6 remains the most commonly utilized salt in the industry today.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, or EV driving simulation test, please feel free to contact us.
