Na batteries (SIBs) are considered a powerful complement to lithium-ion batteries due to their abundant sodium resources and lower production costs, showing immense potential especially in energy storage technology. As a type of secondary (rechargeable) battery, sodium-ion batteries function primarily through the movement of sodium ions between the positive and negative electrodes, operating on a principle similar to that of lithium-ion batteries. The electrode materials for sodium-ion batteries consist mainly of sodium salts, which are more abundant and cost-effective compared to lithium salts.
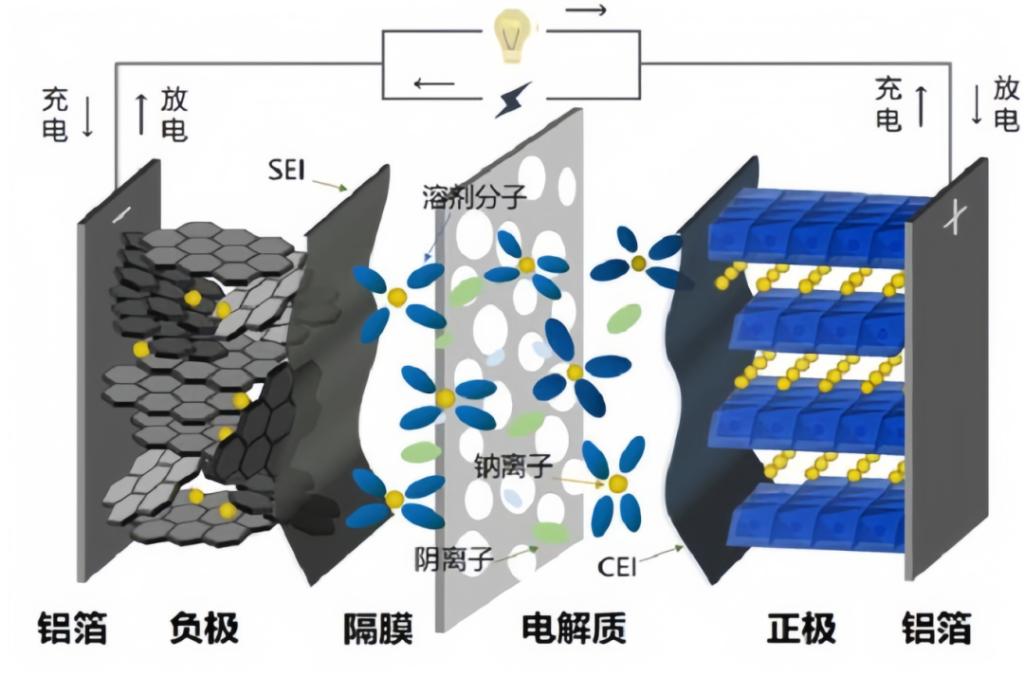
Working Principle of Na Batteries
The working principle of sodium-ion batteries is similar to that of lithium-ion batteries, based on the insertion/extraction (intercalation/deintercalation) process of sodium ions between the positive and negative electrodes to store and release electrical energy. The process primarily includes the following steps:
Charging Process: Under the influence of an external electric field, sodium ions are deintercalated from the cathode material (such as layered oxides or polyanionic compounds), migrate through the electrolyte to the anode (such as hard carbon or sodium titanate), and are embedded into the anode material. Simultaneously, electrons flow from the cathode to the anode via the external circuit.
Discharging Process: Sodium ions are extracted from the anode material and migrate back to the cathode through the electrolyte, while electrons flow back to the cathode through the external circuit, releasing electrical energy.
Na Batteries Electrochemical Reactions
The reactions at the positive and negative electrodes determine the electrochemical performance and energy density of the battery. Typical cathode and anode reactions are as follows:
Cathode Reaction (using NaFePO4 as an example): NaFePO4 <-> FePO4 + Na+ + e-
Anode Reaction (using hard carbon as an example): C + Na+ + e- <-> NaC
The reversibility and stability of these reactions directly impact the charge-discharge efficiency and cycle life of the battery.
Discharge Curve and Characteristics of Na Batteries
A test cell was fabricated using a mixture of Prussian blue-based compounds and layered oxides (such as NaCoO2 or NaFeO2) as the cathode material, with hard carbon used as the anode. Capacity testing was conducted using a standard 0.5C discharge step. The discharge curve of the sodium-ion experimental test cell is shown below:
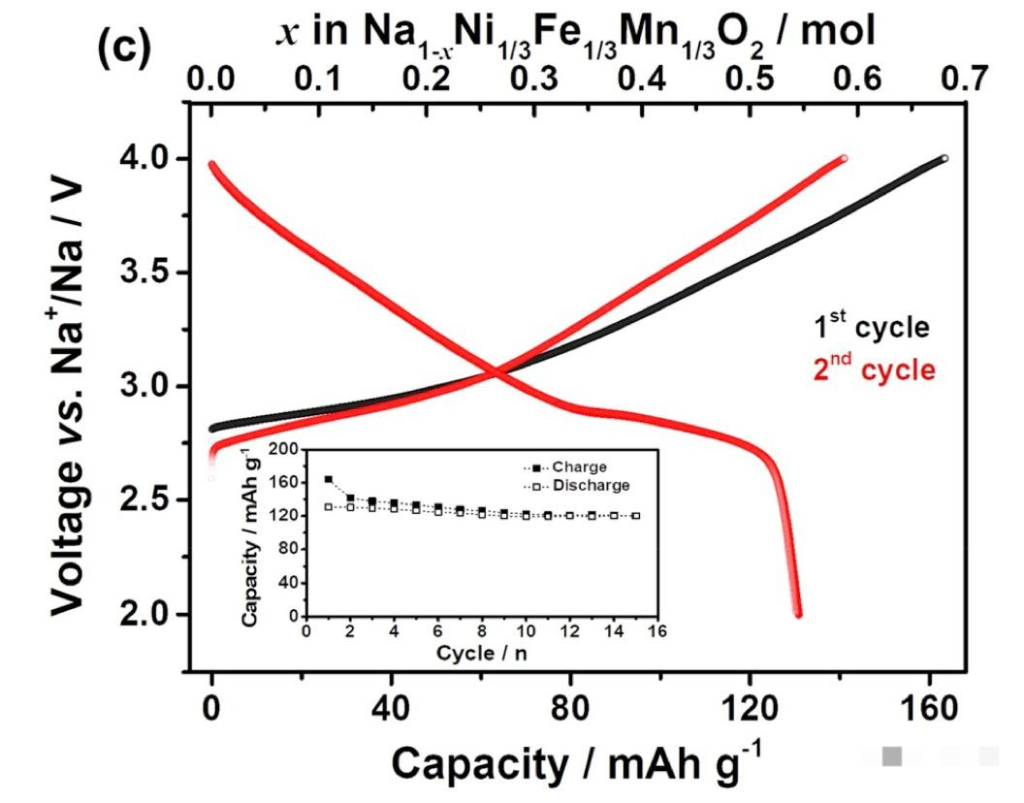
Analysis of Sodium-Ion Test Cell Discharge Characteristics
The charging cut-off voltage for the sodium-ion test cell is 4.0V, the discharging cut-off voltage is 2.0V, and the operating voltage plateau is between 2.5V and 3V. According to the discharge curve:
Steep Voltage Decline: The discharge curve of the sodium-ion battery is characterized by a steep drop. The discharge voltage decreases significantly in the 3.75V to 3V range. The primary discharge operating range is concentrated between 3V and 2.5V. Once the voltage falls below 2.5V, it drops almost vertically to the 2.0V cut-off voltage.
Voltage vs. Capacity Trade-off: The charge-discharge curves indicate that sodium-ion batteries with higher discharge capacities tend to have a lower average cathode discharge voltage.
Material Comparison: Sodium-ion battery cathode materials based primarily on Prussian blue offer excellent rate performance but generally have a moderate cycle life. In contrast, cathode materials based on polyanionic compounds typically exhibit longer cycle lives and higher energy densities, though their rate performance is usually average.
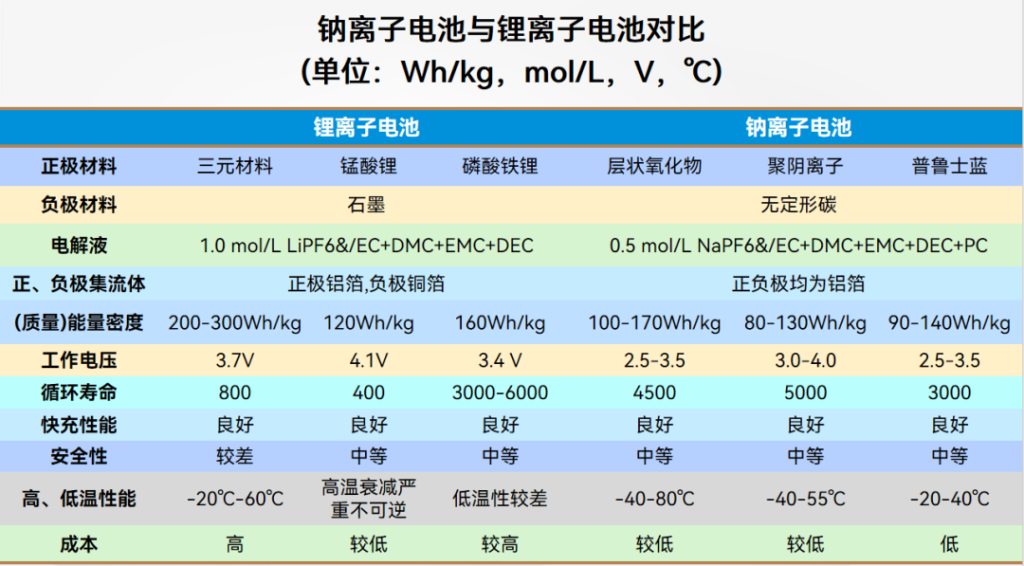
Comparison Between Na batteries and Li batteries
Abundant Resources and Low Cost: Unlike the scarcity of lithium minerals, sodium is significantly more abundant in the Earth’s crust, resulting in lower costs and making it a powerful supplement to lithium-ion batteries. Additionally, both the positive and negative current collectors in sodium batteries use aluminum foil, further reducing costs.
Wide Operating Temperature Range: Sodium-ion batteries perform relatively well in both high and low-temperature environments. In high-temperature settings, they can operate at temperatures exceeding 250°C. In low-temperature environments, they function normally between -40°C and 80°C. Notably, at -20°C, the capacity retention rate of sodium-ion batteries reaches as high as 90%, whereas lithium iron phosphate (LFP) and lead-acid batteries only reach 70% and 48%, respectively. This wide-temperature characteristic ensures stability and reliability under extreme climates, providing critical support for equipment operating in diverse environments.
Fast Charging and Excellent Rate Performance: At the same concentration, sodium-ion battery electrolytes exhibit higher ionic conductivity than lithium-ion electrolytes. Furthermore, sodium ions have lower solvation energy in polar solvents, granting them faster kinetic properties and higher conductivity within the electrolyte.
Longer Cycle Life: Sodium ions have a larger ionic radius; compared to lithium ions, their diffusion speed within materials is slower, which results in less fatigue damage to the electrode materials. Consequently, the cycle life of sodium-ion batteries is longer than that of lithium-ion batteries. If you need more Neware battery cyclers
Enhanced Safety: Sodium-ion batteries have a higher self-ignition temperature and are less prone to safety accidents during overcharging or short-circuiting. They also perform well under extreme conditions, such as crush and nail penetration tests. Additionally, their strong thermal stability allows them to maintain stable performance even in high-temperature environments. Sodium batteries can be stored and transported at zero voltage without safety risks; in the event of a short circuit, they generate less self-heating, eliminating hazards like fire or explosion.
Environmental Friendliness: Since sodium-ion batteries utilize more abundant resources, they offer a greater advantage in environmental protection compared to lithium batteries. Moreover, because most chemical components used in sodium batteries are non-toxic and renewable, the resulting waste poses a lower pollution risk to the environment.
Production Compatibility: Sodium-ion and lithium-ion batteries share similar working principles and material compositions, allowing production experience and equipment to be partially compatible.
Testing and Certification of Na Batteries
As an emerging battery technology, sodium-ion batteries share highly similar structural principles and production processes with lithium-ion batteries. Consequently, the testing and certification of sodium-ion batteries currently follow the same operational procedures as those used for lithium-ion batteries.
01 Sodium-Ion Battery Testing National performance and safety standards specifically for sodium-ion batteries have not yet been released. However, industry specifications have been established to promote development, such as T/CNESA 1006-2021: General Specification for Sodium-ion Secondary Batteries.
Sodium-Ion Battery Testing Items
Sodium-ion battery testing categories include Electrical Performance Testing, Environmental Testing, Mechanical Testing, and Safety Testing.
The specific testing content includes:
Electrical Performance: Room temperature capacity, high-temperature capacity, low-temperature capacity, rate capacity, and insulation resistance/withstand voltage.
Environmental & Reliability: ESD (Electrostatic Discharge), temperature rise, high temperature/high humidity, low air pressure (altitude simulation), salt spray, and dust/water resistance (IP rating).
Mechanical: Vibration, mechanical shock, drop testing, and crush testing.
Safety: Nail penetration, overcharge, short circuit, thermal abuse, and combustion (flammability).
02 Sodium-Ion Battery Certification
Due to their abundant raw materials, low cost, long cycle life, and high safety and reliability, sodium-ion batteries are well-suited as alternatives to lithium-ion batteries for electrochemical energy storage scenarios. Depending on the specific application, the various certifications for sodium-ion batteries can refer to the certification requirements of lithium-ion batteries in energy storage contexts. Corresponding regulatory certifications can be conducted—such as UN Transportation (UN38.3), CB, CE, UL, PSE, and KC—using the standards established for lithium battery energy storage scenarios.
Na Battery Application Scenarios
Household Appliances: The high safety and environmental friendliness of sodium-ion batteries make them the preferred power source for household appliances such as vacuum cleaners and washing machines.
Electric Transportation: Although the charge-discharge rates and energy density of sodium-ion batteries are not as high as those of lithium-ion batteries, their long cycle life and low cost make them suitable for light electric vehicles, including electric bicycles and electric motorcycles.
Large-Scale Energy Storage Systems: The large-scale energy storage capacity and environmental benefits of sodium-ion batteries make them an ideal storage solution for renewable energy facilities, such as solar and wind power plants.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
