Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-2
11. Journal of the American Chemical Society: Kinetics Compensation Mechanism in Cosolvent Electrolyte Strategy for Aqueous Zinc Batteries

Professor Yunhui Huang’s team from Huazhong University of Science and Technology has addressed the inevitable kinetic losses associated with the introduction of co-solvents. They proposed a kinetic compensation mechanism designed to weaken cation-anion interactions and increase the Zn2+ transference number, thereby partially offsetting the kinetic degradation caused by co-solvents. Using an Zn(OTf)2 based aqueous electrolyte containing ethylene carbonate (EC) as a model system, the team demonstrated the effectiveness of this strategy in achieving kinetic compensation and enhancing the electrochemical performance of aqueous zinc secondary batteries.
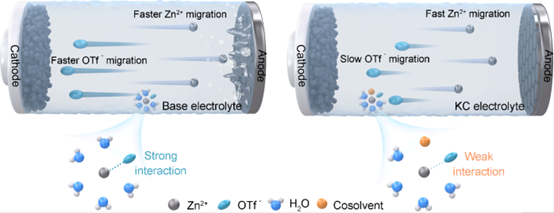
Kinetics Compensation Mechanism in Cosolvent Electrolyte Strategy for Aqueous Zinc Batteries
J. Am. Chem. Soc. 2025, 147, 10, 8607–8617
12. Angewandte Chemie International Edition: Microemulsion Engineering Reconciles Propylene Carbonate Electrolytes and Graphite Anodes for All-Climate Lithium-Ion Batteries

The research team led by Professor Yunhui Huang and Professor Lixia Yuan proposed a novel propylene carbonate (PC)-based microemulsion electrolyte. This electrolyte not only achieves compatibility with graphite anodes but also enables Gr||LFP pouch cells to operate normally across an all-climate temperature range from −60 °C to 100 °C.
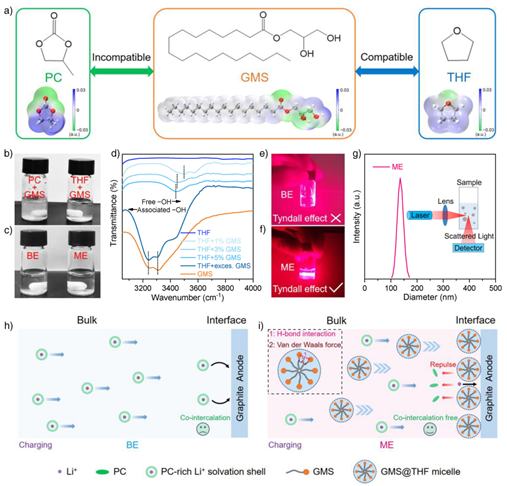
Angew. Chem. Int. Ed. 2025, 64, e202516984
13. Angewandte Chemie International Edition: Anion-Regulated Deposition–Dissolution Chemistry in Acidic Aqueous Indium—MnO2 Batteries

The research teams led by Professor Yunhui Huang (Huazhong University of Science and Technology) and Professor Jing Fu (Tongji University) reported an acidic indium–MnO2 battery (IMB) system. By employing anion regulation of the interfacial chemistry, they achieved a reversible deposition-dissolution reaction at the negative electrode. The In3+/In redox couple of indium (In) possesses a more positive standard reduction potential (–0.34 V vs. SHE), which effectively suppresses parasitic side reactions in acidic media.
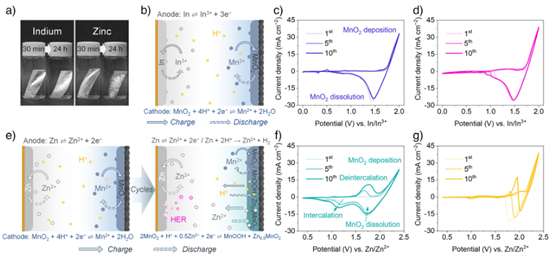
Anion-Regulated Deposition–Dissolution Chemistry in Acidic Aqueous Indium—MnO2 Batteries
Angew. Chem. Int. Ed. 2025, 64, e202512463
14. Advanced Materials: Competitive Ion Coordination in Gel Polymer Electrolytes Enables Decoupling of Mechanical Strength and Ionic Conductivity

The research team led by Prof. Yunhui Huang and Prof. Henghui Xu from Huazhong University of Science and Technology introduced succinonitrile (SN) molecules, which possess a strong affinity for lithium ions, into a poly(methyl methacrylate-co-methacrylamide) (PMAm) gel electrolyte based on hydrogen-bonding functional groups.
By employing a competitive coordination strategy, the lithium ions—originally coordinated with hydrogen-bonding sites on the polymer chains—are released and transferred to the solvent molecules. This process ensures the integrity of the interchain hydrogen-bonding network, thereby maintaining the mechanical robustenss of the gel electrolyte. Furthermore, the formation of interchain hydrogen bonds compresses the interstitial spaces within the polymer framework, forcing solvent molecules and ions into a molecularly aggregated state. This aggregation not only provides fast diffusion channels for Li+ but also enhances the participation of anions in the Li+ solvation shell, ultimately promoting the formation of a stable, inorganic-rich solid electrolyte interphase (SEI).
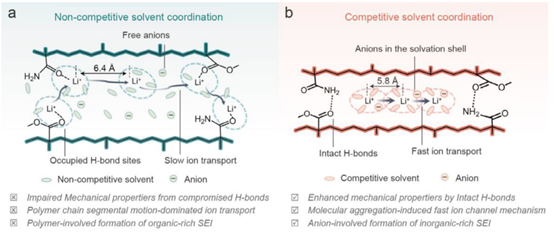
Adv. Mater. 2025, 37, e04625
15. Advanced Materials: Thermoelectric Field Enhanced Sulfur Evolution Kinetics for High Performance Lithium‐Sulfur Batteries

Prof. Yunhui Huang (Huazhong University of Science and Technology) and Prof. Wenyu Zhao (Wuhan Technical University) have designed a Bi0.5Sb1.5Te3/carbon nanotube (BST/CNT) interlayer. This interlayer enhances the durability of lithium-sulfur (Li-S) batteries by providing extensive adsorption sites and leveraging the thermoelectric field generated by the BST thermoelectric material.
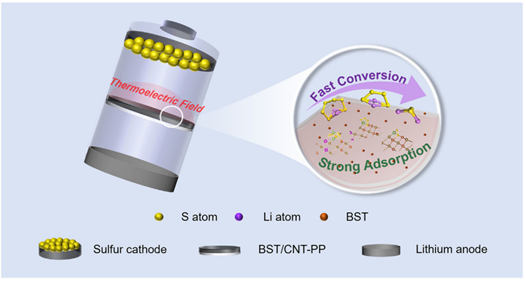
Adv. Mater. 2025, 37, 2500457
16. Energy & Environmental Science: Restoration of Li+ pathways in the [010] direction during direct regeneration for spent LiFePO4
Energy Environ. Sci., 2025, 18, 3750–3760
17. Energy & Environmental Science: Moderate solvation structure of lithium ions for high-voltage lithium metal batteries at −40 ºC

The research team led by Prof. Yunhui Huang and Prof. Henghui Xu from Huazhong University of Science and Technology systematically investigated a series of fluorinated ethyl acetate (FEA) solvents with varying fluorination degrees and/or fluorination sites as electrolyte solvents to reveal the fundamental relationship between molecular structure and their resulting electrochemical properties.
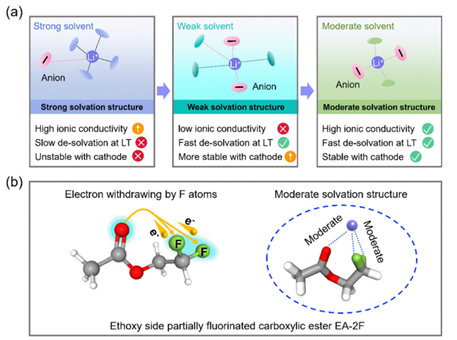
Moderate solvation structure of lithium ions for high-voltage lithium metal batteries at −40 ºC
Energy Environ. Sci., 2025, 18, 786–798
18. Advanced Energy Materials: Dual Regulation of Stability and Kinetics in Iron-Based Mixed Phosphate Cathode for All-Climate Sodium-Ion Batteries
The research team led by Prof. Yunhui Huang (National Distinguished Young Scholar / Changjiang Scholar) and Prof. Chun Fang from Huazhong University of Science and Technology, in collaboration with Yue Xu from Yangtze University (a former team member), proposed an innovative strategy to optimize the local structure of Na4.024Fe2.921(PO4)2P2O7 (NFPP) without introducing heteroatoms.
By modulating the iron valence states during the synthesis process, they developed a non-stoichiometric NFPP-2 (Na4.024Fe2.921(PO4)2P2O7), where the inert Na2 sites are ordered into “structural pillars.” This configuration restricts the lattice volume change to only 4.18% during desodiation, thereby significantly enhancing the structural stability of the material.
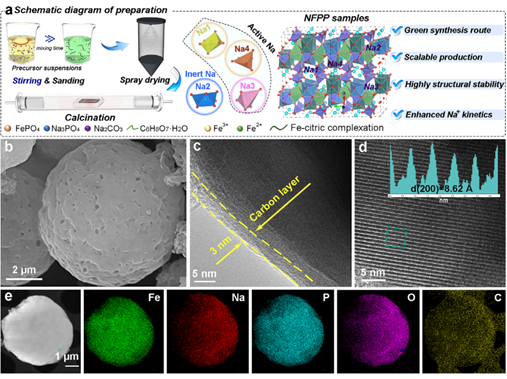
Adv. Energy Mater. 2025, e04854
19. ACS Energy Letters: Recrystallization-Driven Quasi-Spherical Prussian Blue Analogs with High Tap Density and Crystallinity for Sodium-Ion Batteries

The collaborative team led by Yun Qiao and Yang Liu (Shanghai University), Shulei Chou(Wenzhou University), and Yunhui Huang (Huazhong University of Science and Technology) has proposed a recrystallization-driven strategy for the synthesis of monoclinic Prussian blue analogues (CFHCF). The resulting material exhibits high crystallinity and a high tap density (0.992 g cm⁻³).
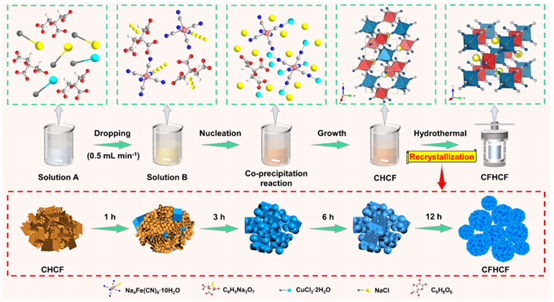
ACS Energy Lett. 2025, 10, 4, 1751–1761
20. Science Bulletin: Anion-mediated electrolyte engineering unlocks high-energy-density and long-cycling sulfur-based batteries at ultra-low N/P ratio

The research team led by Prof. Yunhui Huang and Prof. Zhen Li constructed a SPAN||Gr battery system with an N/P ratio of 0.6, achieving successful cycling through a simple in-situ prelithiation method. Concurrently, they designed an anion-mediated electrolyte (LiFSI/DME/HFE), which is essentially a localized high-concentration electrolyte (LHCE). This electrolyte effectively suppresses the dissolution and shuttling of polysulfides, prevents solvent co-intercalation into the graphite anode, and exhibits excellent compatibility with lithium metal by inhibiting dendrite growth and various parasitic side reactions.
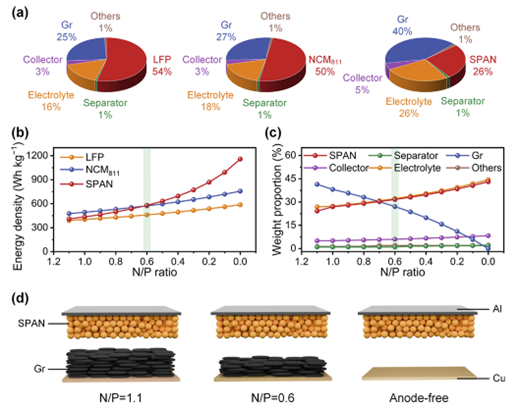
Science Bulletin
Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-1
Related News:
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-1
- Prof. Yunhui Huang’s Group Leads the Way in Battery Innovation: Key Research Highlights (2025)-3
- Lithium Ion vs Lithium Polymer: A Comprehensive Comparison Guide for 2024
- AA Lithium Batteries: Comprehensive Guide 2024 post

![Restoration of Li+ pathways in the [010] direction during direct regeneration for spent LiFePO4](https://www.newarebts.net/wp-content/uploads/Restoration-of-Li-pathways-in-the-010-direction-during-direct-regeneration-for-spent-LiFePO4-1.png)
![Restoration of Li+ pathways in the [010] direction during direct regeneration for spent LiFePO4](https://www.newarebts.net/wp-content/uploads/Restoration-of-Li-pathways-in-the-010-direction-during-direct-regeneration-for-spent-LiFePO4-2.png)