Sharing the testing procedures for coulombic efficiency of lithium copper batteries and LSV.
Source: WeChat Official Account “Brother Radish” 萝卜大师兄 Lbdsx and Google Gemini.
Today I’m sharing the testing procedures for the coulombic efficiency (CUE) and LSV of lithium-copper batteries.
What is LSV?
In the field of lithium metal battery research, LSV (Linear Sweep Voltammetry) for lithium-copper (Li-Cu) asymmetric cells is primarily used to evaluate the electrochemical stability window of the electrolyte and to observe the lithium nucleation process.
Unlike a standard charge/discharge test, LSV involves sweeping the potential at a constant rate to observe where the electrolyte begins to decompose or where lithium begins to plate onto the copper substrate.
Here is a breakdown of what LSV reveals in a Li-Cu system:
1. Determining the Electrochemical Window
The most common use of LSV in a Li-Cu (or Li-Stainless Steel) cell is to determine the oxidative stability of the electrolyte.
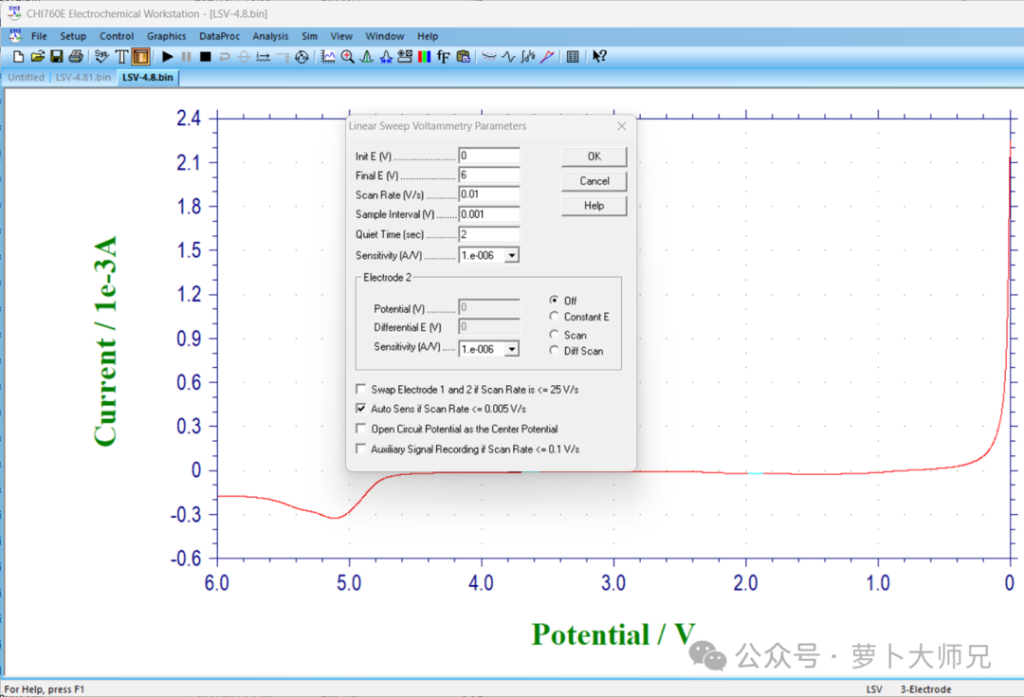
Procedure: The potential is swept from the open-circuit voltage (OCV) upward to high voltages (e.g., 5.0 V or 6.0 V).
Observation: Researchers look for a sudden increase in current, which indicates the onset of electrolyte oxidation.
Significance: This tells you the maximum voltage the battery can safely operate at without the electrolyte “breaking down” on the cathode side.
2. Studying Lithium Nucleation and Plating
When the voltage is swept in the negative direction (toward or below 0 V vs. Li/Li+), LSV helps analyze how lithium ions deposit onto the copper foil.
Nucleation Overpotential: The “dip” in the voltage curve before it stabilizes during lithium plating. A smaller overpotential usually indicates a more “lithium-friendly” (lithophilic) surface or a better electrolyte interface.
Plating Current: The magnitude of the current response shows the kinetics of the lithium deposition process.
3. Key Testing Parameters
Scan Rate: Typically very slow, such as 0.1 mV/s to 1 mV/s. Fast scan rates can mask the true decomposition potential due to capacitive currents.
Working Electrode: Copper foil (Cu) or Stainless Steel (SS).
Counter/Reference Electrode: Lithium metal (Li).
4. Technical Terms Summary
Oxidative Decomposition: The breakdown of the electrolyte at high voltage.
Asymmetric Cell: A cell using two different electrodes (Li and Cu).
Onset Potential: The voltage at which a specific electrochemical reaction begins.
5.LSV Testing Procedure
The LSV measurement for solid electrolytes, separators, or electrolyte solutions (solid polymer electrolytes are also tested after standing at 60℃ for 12 hours) is performed by assembling asymmetric lithium/steel cells, as shown in Figure 1. The start and stop voltages are set to 0-6 V (generally 2-6 V is sufficient for faster measurement), the scan rate is 10 mV/s, and the standing and recording times can be left unchanged (if you’re worried about too much data, you can change the recording time to 1). The sensitivity can also be left unchanged (in Chenhua’s electrochemical workstation, just check the bottom option). This test is very simple.
What is Coulombic Efficiency?
In the context of lithium metal battery research, the Coulombic Efficiency (CE) of a lithium-copper (Li-Cu) battery—specifically a Li-Cu asymmetric cell—is a critical metric used to evaluate the reversibility of lithium plating and stripping.
Since copper foil does not contain any lithium, every atom of lithium that is deposited (plated) on it must come from the lithium metal counter electrode. The CE measures how much of that lithium can successfully be removed (stripped) back off during the next half-cycle.
1. How Coulombic Efficiency Is Measured
In a Li-Cu cell, the test is typically performed using a galvanostatic (constant current) method:
Plating Step: A fixed amount of lithium (e.g., 1.0 mAh/cm2) is deposited onto the copper foil by applying a negative current.
Stripping Step: The current is reversed to remove the lithium. The stripping continues until the voltage reaches a specific cutoff (usually 0.5 V or 1.0 V vs. Li/Li+), indicating all accessible lithium has been removed.
2. The Formula of Coulombic Efficiency
The Coulombic Efficiency is calculated as the ratio of the capacity recovered during stripping to the capacity applied during plating:
CE (%) = (Stripping Capacity / Plating Capacity) x 100%
3. Why Is Coulombic Efficiency Never 100%?
In a perfect world, CE would be 100%. However, in real batteries, lithium is lost during every cycle due to:
SEI Formation: Some lithium reacts chemically with the electrolyte to form the Solid Electrolyte Interphase (SEI) layer.
“Dead” Lithium: During stripping, some lithium becomes electrically isolated from the copper foil (often due to dendritic growth), leaving behind “dead lithium” that can no longer participate in the reaction.
4. Common Testing Protocols of Coulombic Efficiency
Standard CE Test: A simple cycle-by-cycle calculation.
Aurbach Method: A more rigorous protocol involving an initial “reservoir” of lithium being plated, followed by several cycles of partial stripping and plating, and finally a full strip to calculate an average CE. This is considered more accurate for high-performance systems.
5. Coulombic Efficiency Significance in Research
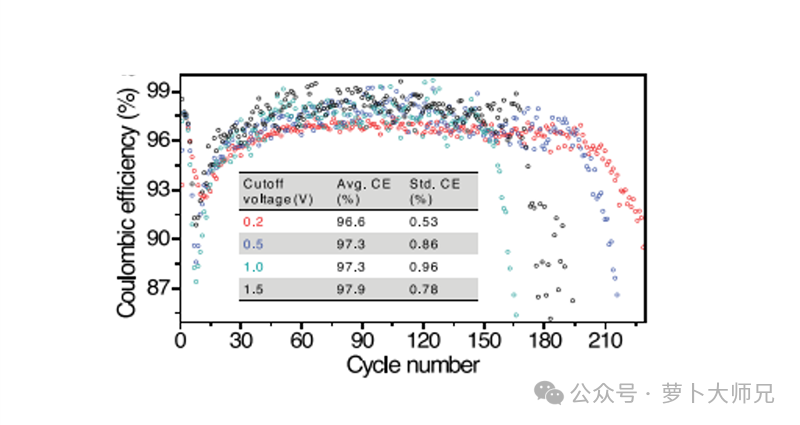
Electrolyte Screening: High CE (usually >99.5% for commercial viability) indicates that the electrolyte is stable and forms a high-quality SEI.
Anode Protection: If you are testing a protective coating on the copper foil, an increase in CE suggests the coating successfully reduces side reactions or dendrite formation.
6. Lithium-Copper Battery Coulombic Efficiency Process
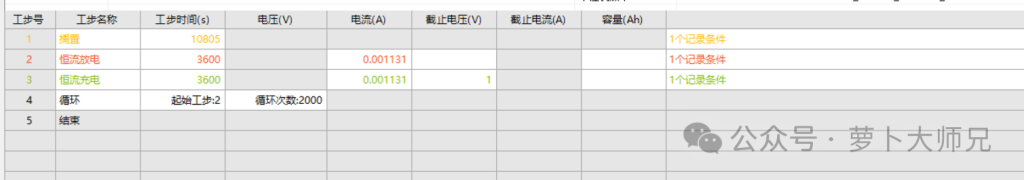
The process for lithium-copper batteries is shown in Figure 2. First, discharge, then charge. The charging cutoff voltage can be set to 0.5V, 1V, or 1.5V, depending on the battery materials used. The data obtained are the cycle count and coulombic efficiency.
Additionally, the polarization voltage can be compared using a voltage-capacity curve. The current is calculated by multiplying the current density by the area. Here, the current density is 1 mA/cm², and the area is calculated based on the smaller of the lithium and copper sheets. Our copper sheet is smaller, with a diameter of 1.2cm. Furthermore, if the coulombic efficiency is low in the first few cycles, a smaller current can be used to activate the battery before further testing. We are using Neware battery testers.
Due to the limited knowledge and English level is inevitable errors and omissions, if there are errors or infringement of the text, please contact me as soon as possible by private letter, I will immediately be corrected or deleted.
Related News:
- Design, Assembly, and Testing of Full Coin Cells: Tutorials and Case Studies 2026 post
- Electrochemical Test Techniques for Lithium-ion Batteries: CV, EIS
- Lithium Ion vs Lithium Polymer: A Comprehensive Comparison Guide for 2024
- How to determine the charge/discharge cutoff voltage of a lithium-ion battery?
